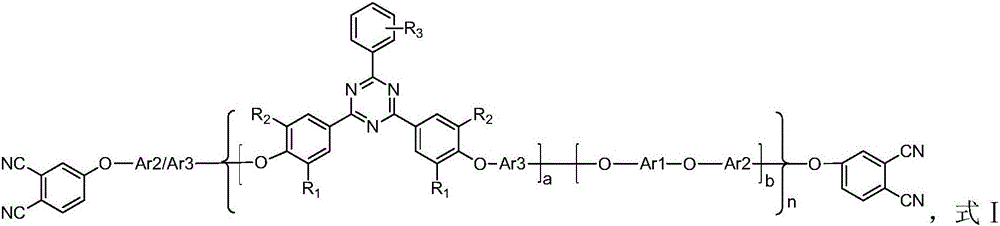
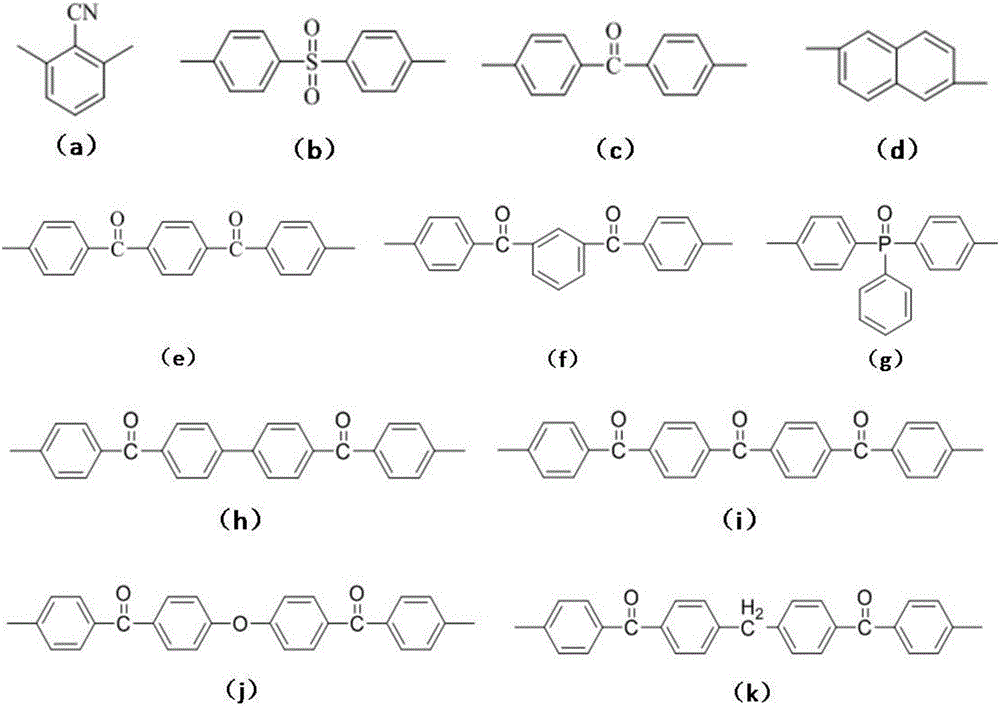
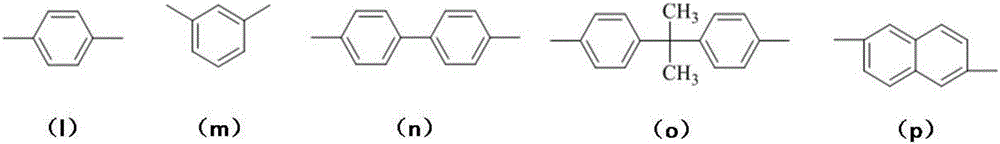
Bisphthalonitrile resin fiber enhanced material with triphenyl sym-triazine structure and preparation method thereof
A technology of bis-phthalonitrile resin and phthalonitrile resin, which is applied in the field of bis-phthalonitrile resin reinforced fiber material and its preparation, can solve the problem of low heat resistance of resin, not involving preparation and use, etc. problem, to achieve the effect of improving heat resistance, widening application, and high heat resistance level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of the phthalonitrile resin containing triaryl-s-triazine structure shown in embodiment 1 formula a (referred to as resin A)
[0045]
[0046] Among them, the Ar3 group selects (q) structure, R1~R6 groups are all -H, a=1, b=0, the molecular weight of the polymer is 1820 as detected by GPC, and the number n of repeating structural units is 2.4.
[0047] The preparation method is as follows:
[0048] (1) Add 47.6492g of 4-(4-hydroxyphenyl)-2,3-phthalazin-1-one, 34.5108 g 2,4-bis(4-fluorophenyl)-6-phenyl-1,3,5-triazine, 35.8572g anhydrous potassium carbonate, 100mL N-methylpyrrolidone and 50mL toluene; reflux at 150℃ with water After 3 hours, toluene was distilled off; continue to heat up to 190°C and react for 7 hours. During the reaction, add solvent according to the viscosity of the reaction system until the viscosity of the reaction system no longer increases. The amount of added solvent is 3 mL; cool to room temperature to obtain The reaction solu...
Embodiment 2
[0053] The preparation of the phthalonitrile resin containing triaryl-s-triazine structure shown in embodiment 2 formula b (abbreviated as resin B)
[0054]
[0055] Among them, R1~R3 groups are all -H, a=1, b=0, Ar3 group adopts (n) structure, the molecular weight of the polymer is 1297 as detected by GPC, and the number n of repeating structural units is 1.6.
[0056] The preparation method is as follows:
[0057] (1) Add 1.4896g of biphenyldiphenol and 1.3804g of 2,4-bis(4-fluorophenyl)-6-phenyl -1,3,5-Triazine, 1.3456g anhydrous potassium carbonate, 5mL sulfolane and 20mL toluene; after refluxing with water at 150°C for 3 hours, distill the toluene; Additional solvent was added until the viscosity of the reaction system no longer increased, and the amount of added solvent was 3 mL; cooled to room temperature to obtain a reaction solution of an oxophenoxide-capped intermediate.
[0058] (2) Add 1.1620 g of 4-nitrophthalonitrile to the reaction solution of the phenoxy s...
Embodiment 3
[0063] The preparation of the phthalonitrile resin containing triaryl-s-triazine structure shown in embodiment 3 formula c (abbreviated as resin C)
[0064]
[0065] Wherein, R1~R3 groups are all -H, a=0.5, b=0.5, Ar3 group adopts (n) structure, Ar2 adopts (l) structure, and Ar1 adopts (a) structure. The molecular weight of the polymer was detected by GPC as 1980, and the number of repeating structural units n was 4.4.
[0066] The preparation method is as follows:
[0067] (1) Add 1.1173g of biphenol, 0.4404g of hydroquinone, 0.5564g of 2,6-difluorobenzonitrile and 1.3804 g 2,4-bis(4-fluorophenyl)-6-phenyl-1,3,5-triazine, 1.9308g anhydrous potassium carbonate, 10mL dimethyl sulfoxide and 20mL toluene; reflux at 150°C with water After 3 hours, toluene was distilled off; continue to heat up to 190°C and react for 7 hours. During the reaction, add solvent according to the viscosity of the reaction system until the viscosity of the reaction system no longer increases. The am...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| bending strength | aaaaa | aaaaa |
| shear strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com