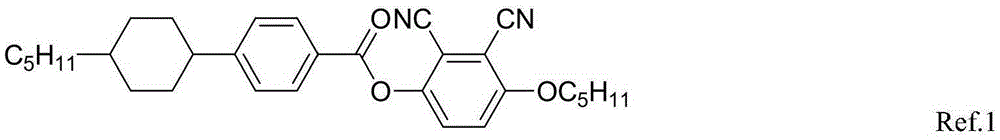
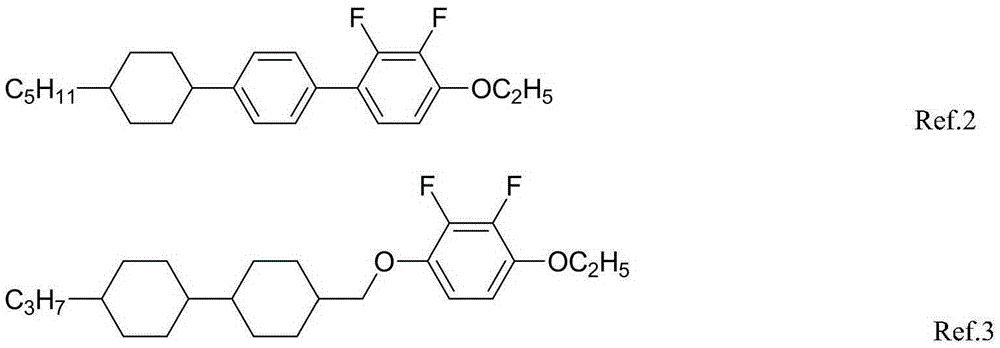
Liquid crystal compounds having negative dielectric anisotropy and applications thereof
An anisotropic, liquid crystal composition technology, applied in organic chemistry, liquid crystal materials, nonlinear optics, etc., can solve the problems of poor mutual solubility, high viscosity, and poor photostability of liquid crystal monomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Compound Ⅰ-A-7 synthetic route is as follows:
[0088]
[0089] 1) Synthesis of Compound B
[0090] In a 500ml three-necked flask, add 10g compound A (2,3-difluoro-4-ethoxyphenylboronic acid), 9.7g 2,3-difluorobromobenzene, 100ml toluene, 50ml ethanol, 50ml water, 21.2g sodium carbonate , under the protection of nitrogen, add 0.3g Pd(PPh3)4, heat to reflux for 6h, separate liquid, wash with water, column chromatography gives white solid compound B 11.5g, GC>97%, yield: 85.3%
[0091] 2) Synthesis of Compound C
[0092] In a 250ml three-neck flask, add 6.7g of compound B and 100ml of anhydrous tetrahydrofuran, under nitrogen protection, cool down to -78°C, add 10.5ml of n-butyllithium n-hexane solution (2.4mol / L) dropwise, keep stirring for 2h, drop Add a mixed solution of 1.8g tetrahydropyran-2-one (CAS#542-28-9) and 10ml anhydrous tetrahydrofuran, and control the temperature to -65~-70°C. After the dropwise addition, keep stirring for 1h , and then the reaction s...
Embodiment 2
[0109] The synthetic route of compound Ⅰ-E-7 is as follows:
[0110]
[0111] 1) Synthesis of Compound D
[0112] Into a 500ml three-necked flask, add 13.5g of compound B and 150ml of anhydrous tetrahydrofuran, under nitrogen protection, cool down to -78°C, add 20.8ml of n-butyl lithium in n-hexane solution (2.4mol / L) dropwise, after the dropwise addition, Continue to keep stirring at -78°C for 1.5h, then add dropwise a mixture of 13g of triisobutyl borate and 25ml of anhydrous tetrahydrofuran, after the dropwise addition, keep at -78°C, stir for 0.5h, naturally warm to room temperature, and post-process to obtain Off-white solid, compound D 11g, HPLC>95%, yield: 70%.
[0113] 2) Synthesis of Compound E
[0114] In a 500ml three-necked flask, add 11g of compound D, 150ml of dichloromethane, and 50ml of dioxane, cool down to 0°C, add 12ml of 30% hydrogen peroxide dropwise, and control the temperature within 10°C. After the dropwise addition, stir at room temperature for 3h...
Embodiment 3
[0120] Compound I-A-7, Compound I-A-1, Compound I-E-7, Compound V were mixed with the host liquid crystal (host) at a ratio of 10:90, and the liquid crystal parameters of each compound were tested by extrapolation method as shown in Table 2. Show:
[0121]
[0122] Table 2
[0123] Δn Δε gamma 1 Ⅰ-A-7 0.155 -9.8 291 Ⅰ-A-1 0.128 -8.2 208 Ⅰ-E-7 0.156 -12.2 454 Ⅴ 0.152 -9.6 439 host 0.08 5.0 116
[0124] Compared with compound V, the compound of the present invention has a low γ1 value when the dielectric anisotropy is close; and when the γ value is close, it has a larger absolute value of the dielectric anisotropy; the above examples The test data results show that the liquid crystal compound provided by the present invention can achieve the invention purpose of large dielectric anisotropy and low viscosity, so that the liquid crystal composition comprising the present invention has a faster response speed and a lower th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com