Fine silver particle dispersion, fine silver particles, and method for producing same
A manufacturing method and dispersion technology, which can be used in cable/conductor manufacturing, manufacturing tools, conductive materials dispersed in non-conductive inorganic materials, etc. The effect of sinterability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
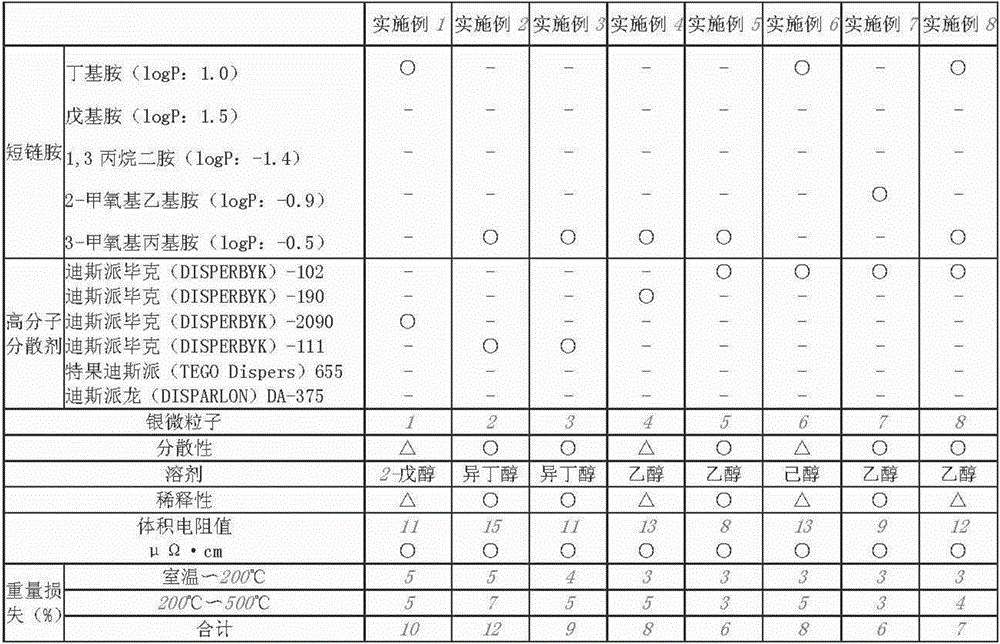
Examples
Embodiment 1
[0144] Mix 200 ml of toluene (first grade reagent manufactured by Wako Pure Chemical Industries, Ltd.) and 11 g of butylamine (first grade reagent manufactured by Wako Pure Chemical Industries, Ltd., carbon number: 4, logP: 1.0), and stir with a magnetic force (the molar ratio of the added amine relative to the silver is 2.5). While stirring, 10 g of silver nitrate (special grade reagent manufactured by Toyo Chemical Industry Co., Ltd.) was added thereto, and after the silver nitrate was dissolved, 10 g of DISPERBYK-2090 and hexanoic acid were added as a polymer dispersant (Reagent special grade manufactured by Wako Pure Chemical Industries Co., Ltd.) 10 g.
[0145] A 0.02 g / ml sodium borohydride aqueous solution prepared by adding 1 g of sodium borohydride (manufactured by Wako Pure Chemical Industries, Ltd.) to 50 ml of ion-exchanged water was added dropwise thereto to obtain a solution containing silver fine particles. After stirring for 1 hour, 200 ml of methanol (a speci...
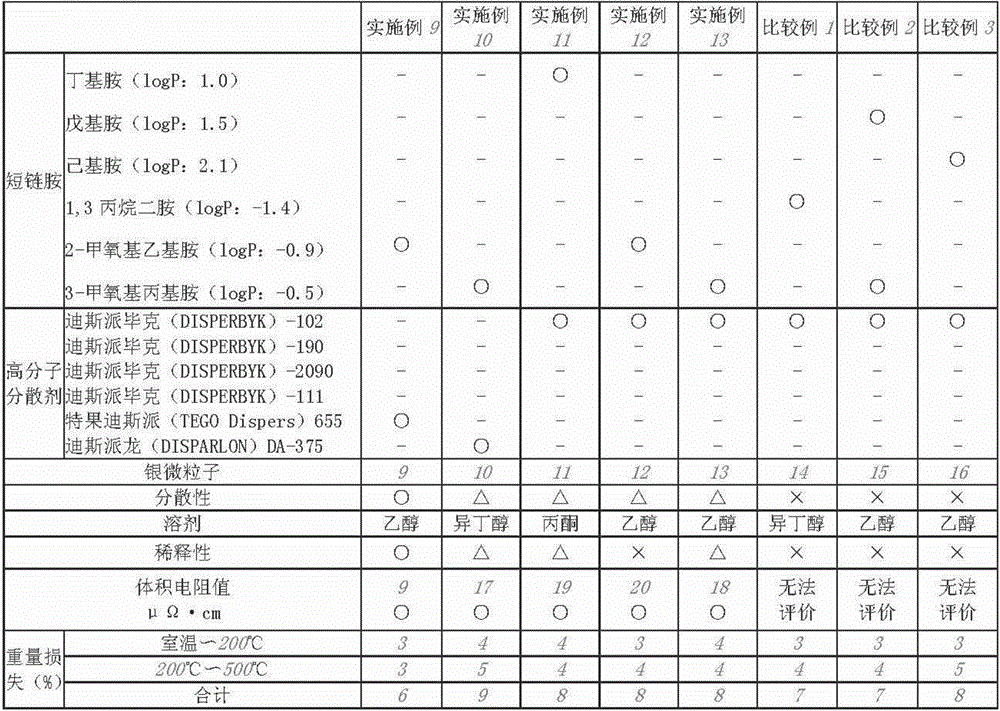
Embodiment 2
[0157] 200 ml of toluene (reagent grade 1 manufactured by Wako Pure Chemical Industries Co., Ltd.) and 3-methoxypropylamine (reagent grade 1 manufactured by Wako Pure Chemical Industries Ltd., carbon number: 4, logP: -0.5) 13.4 g was mixed and stirred well with a magnetic stirrer (the molar ratio of the added amine to silver was 2.5). While stirring, 10 g of silver nitrate (special grade reagent manufactured by Toyo Chemical Industry Co., Ltd.) was added thereto, and after the silver nitrate was dissolved, 10 g of DISPERBYK-111 and hexanoic acid were added as a polymer dispersant (Reagent special grade manufactured by Wako Pure Chemical Industries Co., Ltd.) 10 g. A 0.02 g / ml sodium borohydride aqueous solution prepared by adding 1 g of sodium borohydride (manufactured by Wako Pure Chemical Industries, Ltd.) to 50 ml of ion-exchanged water was added dropwise thereto to obtain a solution containing silver fine particles. After stirring for 1 hour, 200 ml of methanol (a special...
Embodiment 3
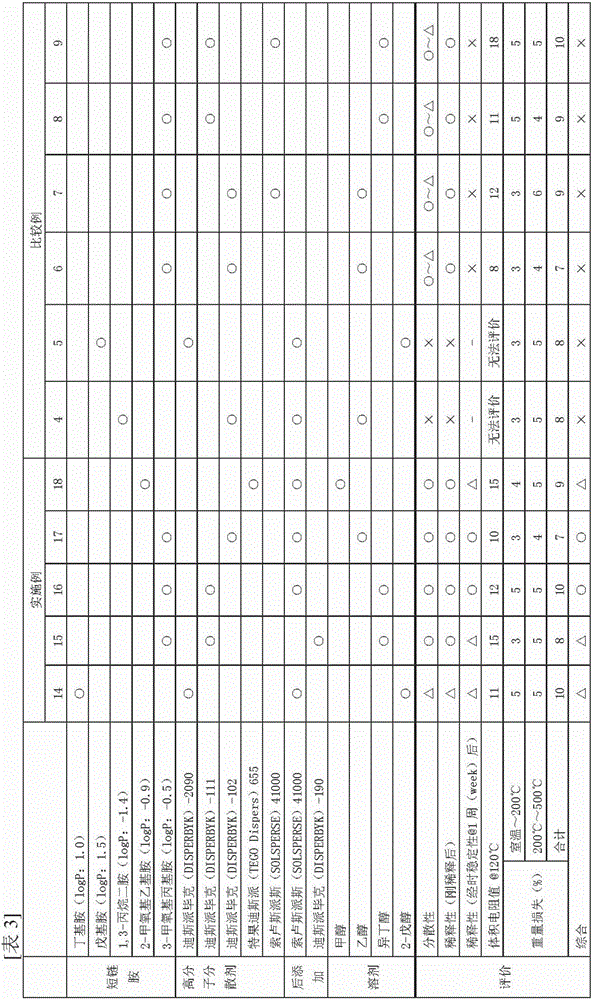
[0159]8.9 g of 3-methoxypropylamine (reagent grade 1 manufactured by Wako Pure Chemical Industries, Ltd., carbon number: 4, logP: -0.5) and 0.3 g of Despex as a polymer dispersant (DISPERBYK)-111 was mixed and fully stirred with a magnetic stirrer to generate an amine mixed solution (the molar ratio of the added amine to silver was 10). Then, while stirring, 3.0 g of silver oxalate was added. After the silver oxalate was added, stirring was continued at room temperature to change the silver oxalate into a viscous white substance, and the stirring was terminated when the change in appearance was found to be complete (first step).
[0160] The obtained mixed solution was transferred to an oil bath, and heated and stirred at 120°C. The reaction accompanied by the generation of carbon dioxide starts immediately after the stirring is started, and then the stirring is continued until the generation of carbon dioxide is completed, thereby obtaining a suspension in which silver fine ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com