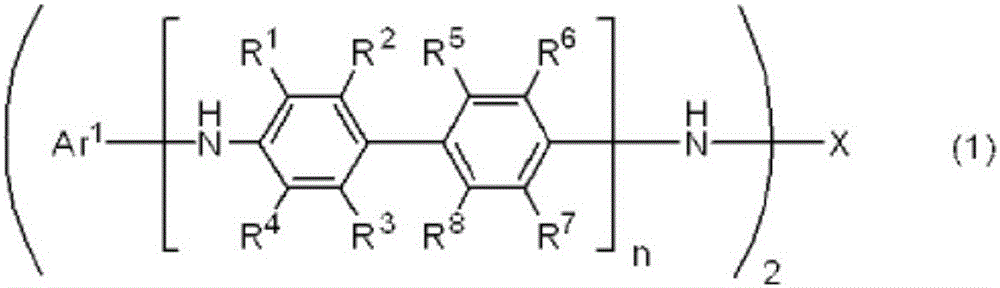
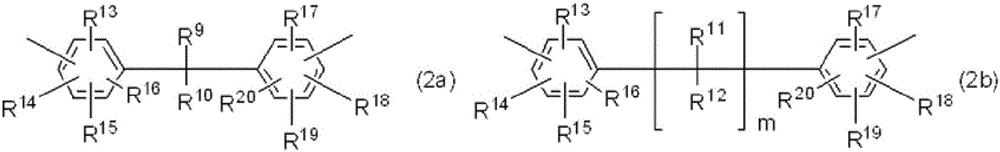
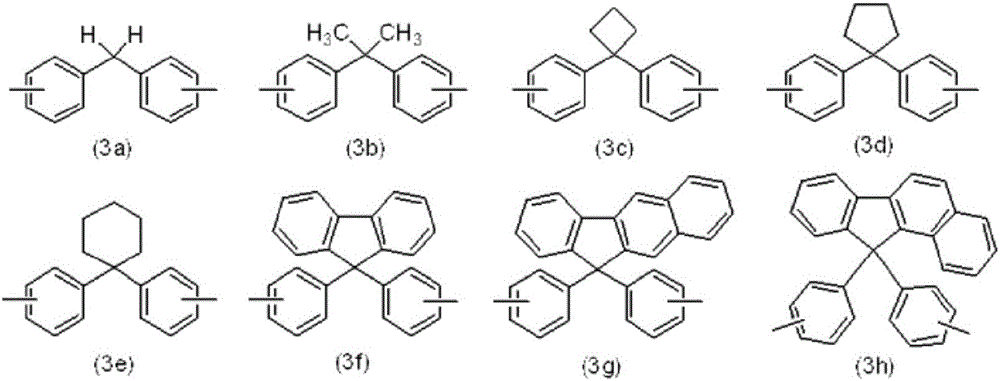
Charge-transporting varnish
A technology of charge transport and varnish, which is applied in circuits, electric light sources, photovoltaic power generation, etc., can solve unconfirmed problems and achieve high charge transport effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0186] Synthesis examples and examples are given below to describe the present invention more specifically, but the present invention is not limited to the following examples. In addition, the apparatuses used are as follows.
[0187] (1) 1 H-NMR: ECX-300 manufactured by JEOL Ltd.
[0188] (2) LC / MS: Waters (Waters) Co., Ltd., ZQ2000
[0189] (3) Substrate cleaning: Substrate cleaning device manufactured by Choshu Sangyo Co., Ltd. (decompression plasma method)
[0190] (4) Coating of varnish: Mikasa (ミカサ) Co., Ltd. spin coater MS-A100
[0191] (5) Film thickness measurement: Micro shape measuring machine Suifcorder (Surfcorder) ET-4000 manufactured by Kosaka Laboratory
[0192] (6) Production of EL elements: Multi-functional vapor deposition system C-E2L1G1-N manufactured by Choshu Sangyo Co., Ltd.
[0193] (7) Measurement of luminance, etc. of EL elements: (Yes) I-V-L measurement system manufactured by Tech World Inc. (Tech World Inc.)
[0194] [1] Synthesis of Arylamin...
Synthetic example 1
[0195] [Synthesis Example 1] Synthesis of Compound 1
[0196] [chemical 18]
[0197]
[0198] To a toluene suspension (90 mL) of 4-bromo-4′-iodobiphenyl (8.98 g, 25 mmol, manufactured by Tokyo Chemical Industry Co., Ltd.), aniline (2.56 g, 27.5 mmol), was added Pd(PPh 3 ) 4(1.44g, 1.25mmol) and t-BuONa (2.88g, 30mmol) were replaced with nitrogen, and heated to reflux for 10 hours. After the reaction, let cool to room temperature, and filtered with celite. The filtrate was concentrated, the obtained crude product was purified by silica gel column chromatography (eluent: toluene), and the fraction containing Compound 1 was concentrated. A mixed solvent of ethanol / toluene (3:1 (w / w)) was added to the obtained crude product, and it was dissolved under heating and reflux. After cooling to room temperature, the precipitated solid was filtered to obtain Compound 1 (6.96 g, yield 86%) as a light brown solid. 1 The measurement results of H-NMR and LC / MS are shown below.
[019...
Synthetic example 2
[0201] [Synthesis Example 2] Synthesis of Arylamine Derivative H1
[0202] [chemical 19]
[0203]
[0204] Xylene suspension of compound 1 (1.95 g, 6.0 mmol) prepared in Synthesis Example 1 to 9,9-bis(4-aminophenyl)fluorene (1 g, 2.87 mmol, manufactured by Tokyo Chemical Industry Co., Ltd.) (10mL), add Pd(PPh 3 ) 4 (166mg, 0.14mmol), t-BuONa (0.66g, 6.89mmol), after being replaced with nitrogen, heated to reflux for 4 hours. Then, Compound 1 (0.37 g, 1.1 mmol) and t-BuONa (0.11 g, 1.21 mmol) obtained in Synthesis Example 1 were added, followed by heating under reflux for 6 hours. After completion of the reaction, let cool to room temperature, add chloroform (40 mL) and water (40 mL), and stir at room temperature for 30 minutes. The insoluble solid was collected by filtration, dissolved in THF, and filtered through celite. The filtrate was concentrated, and the obtained crude product was purified by silica gel column chromatography (eluent: hexane / ethyl acetate (1 / 1 (v / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com