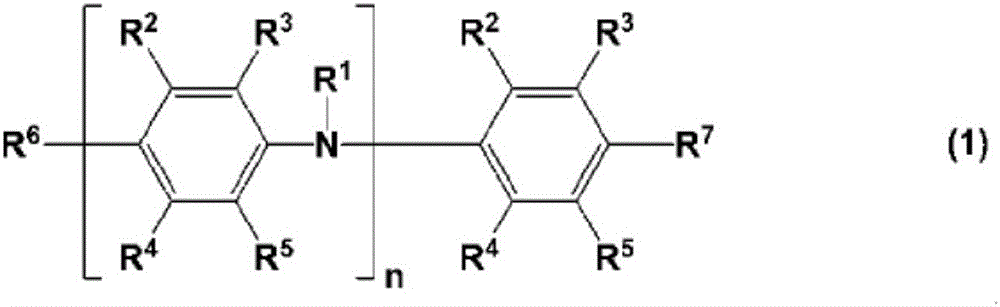
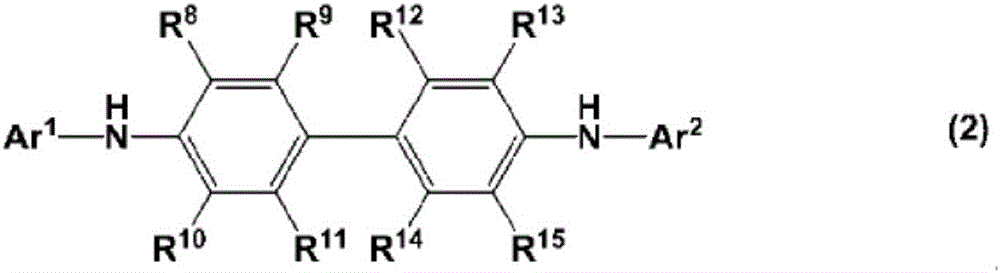
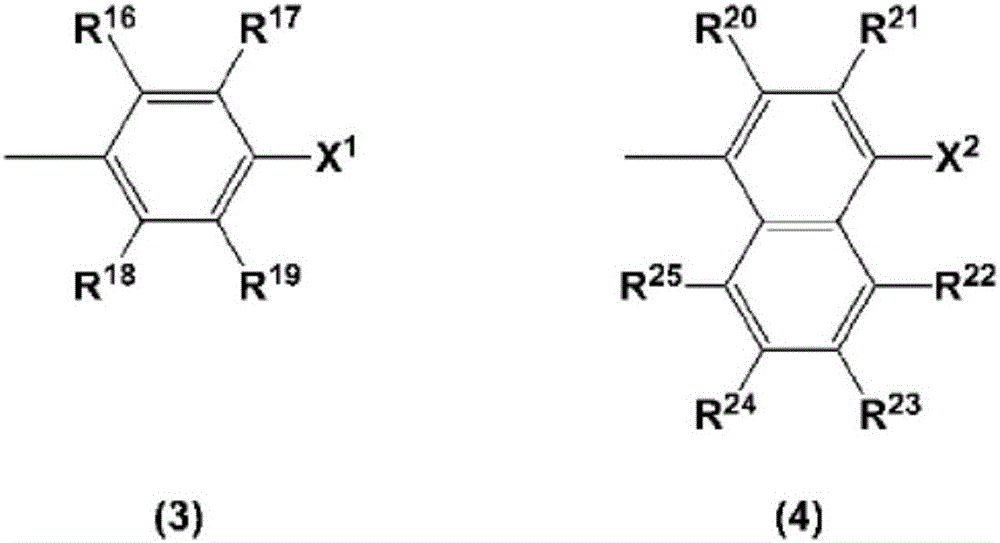
charge transport varnish
A charge-transporting, varnish-based technology applied in the field of organic EL to improve light extraction efficiency, lower drive voltage, and ensure color reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0152] The following examples and comparative examples are given to describe the present invention more specifically, but the present invention is not limited to the following examples. In addition, the apparatuses used are as follows.
[0153] (1) 1 H-NMR measurement: High-resolution nuclear magnetic resonance equipment manufactured by Barian
[0154] (2) Substrate cleaning: Substrate cleaning equipment manufactured by Changzhou Sangyo Co., Ltd. (decompression plasma method)
[0155] (3) Coating of varnish: spin coater MS-A100 manufactured by Mikasa Co., Ltd.
[0156] (4) Film thickness measurement: Micro shape measuring machine Surfcorder ET-4000 manufactured by Kosaka Laboratories Co., Ltd.
[0157] (5) Transmittance measurement: Visible ultraviolet absorption spectrometer UV-3100PC manufactured by Shimadzu Corporation
[0158] (6) Manufacture of EL elements: Multifunctional vapor deposition system C-E2L1G1-N manufactured by Changzhou Sangyo Co., Ltd.
[0159] (7) Meas...
Synthetic example 1
[0161] [Synthesis Example 1] Synthesis of Aniline Derivative A (Formula (f))
[0162] [chemical 12]
[0163]
[0164] Add tetrakis(triphenyl 0.5799 g (0.5018 mmol) of phosphine) palladium and 10.13 g (105.40 mmol) of sodium tert-butoxide were stirred at 130° C. for 14 hours under nitrogen.
[0165] Then, the reaction mixture was filtered, saturated brine was added to the filtrate, and after liquid separation treatment, the solvent was distilled off from the organic layer, and the obtained solid was recrystallized using 1,4-dioxane to obtain aniline Derivative A (yield: 22.37 g, yield: 65%).
Synthetic example 2
[0166] [Synthesis Example 2] Synthesis of Aryl Sulfonic Acid C (Formula (8))
[0167] [chemical 13]
[0168]
[0169] In 11.0 g (31.59 mmoles) of fully dried 1-naphthol-3,6-sodium disulfonate, 4.797 g (14.36 moles) of perfluorobiphenyl and 4.167 g (30.15 moles) of potassium carbonate were added successively under a nitrogen atmosphere. ) and 100 mL of N,N-dimethylformamide, and the reaction system was replaced with nitrogen, and stirred at an internal temperature of 100° C. for 6 hours.
[0170] After naturally cooling to room temperature, 500 mL of N,N-dimethylformamide was further added in order to redissolve the arylsulfonic acid C precipitated after the reaction, and the mixture was stirred at room temperature for 90 minutes. After stirring, the solution was filtered to remove potassium carbonate residue and concentrated under reduced pressure. Furthermore, in order to remove remaining impurities, 100 mL of methanol was added to the residue, and the mixture was stirre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com