Glucosyl hesperidin, method for manufacturing same, and application for same
A manufacturing method and technology of hesperetin, which are applied in the directions of skin care preparations, sugar derivatives, pharmaceutical formulations, etc., can solve the problems of not providing glycosyl hesperetin products, etc., and achieve high quality, reduced coloring or odor, The effect of odor reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0043] Hereinafter, although embodiment of this invention is described, these are illustrations of preferred embodiment for carrying out this invention after all, and this invention is not limited to these embodiment.
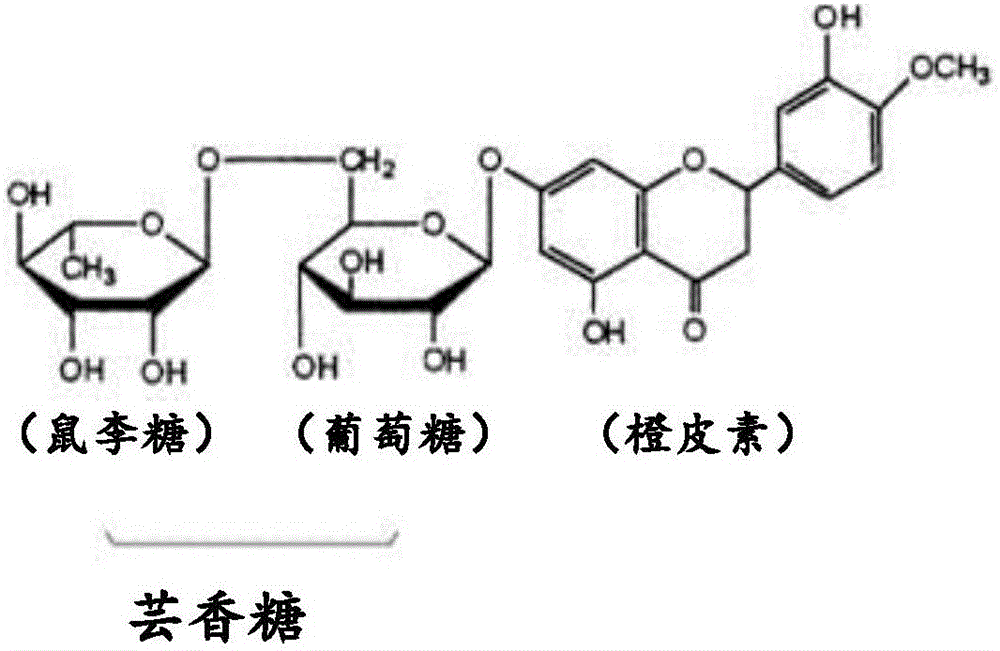
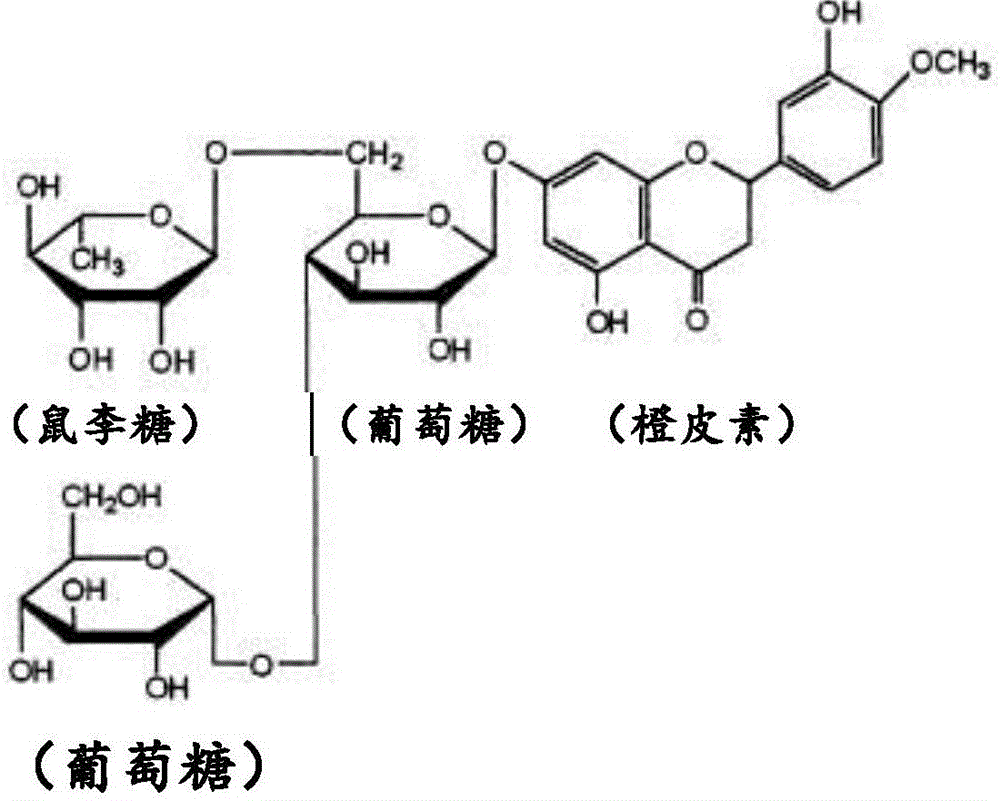
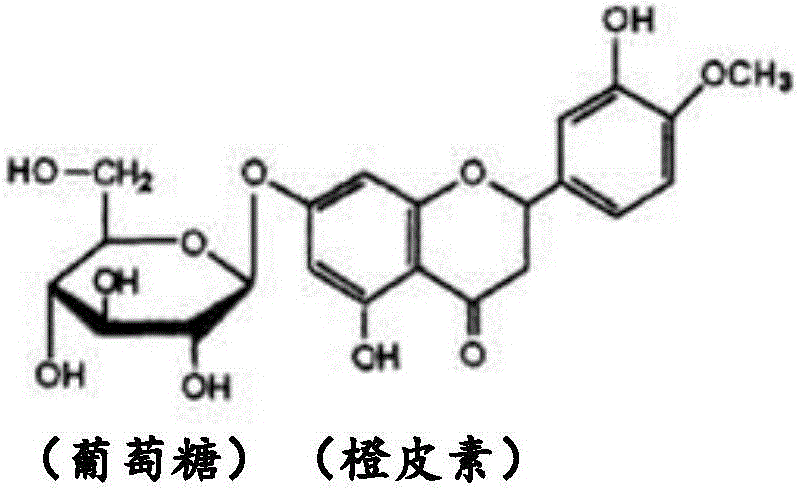
[0044] Glycohesperetin according to the present invention is a novel glycohesperetin having significantly lower off-taste than conventional products, although it has glycohesperetin as a main component in common with conventional products.
[0045] That is, the glycosyl hesperetin in the present invention refers to a compound containing (1) α-glycosyl hesperidin (α-glucosyl hesperidin, etc.) and (2) orange A composition containing one or both of dermatin and 7-O-β-glucosyl hesperetin as the main components, that is, a glycosyl hesperetin mixture, and containing (3) flavonoids such as naringel rutin, diosin, neocitrudin, glucosyl naringing rutin, etc., and (4) trace components such as salts. The total ratio (mass %) of the above-mentioned (1) and (2) components...
Embodiment 1
[0259]
[0260] Warm 4 parts by mass of 1N sodium hydroxide aqueous solution to 80°C, maintain this temperature, add 1 part by mass of hesperidin and 7 parts by mass of dextrin (DE20), dissolve while stirring for 30 minutes, and set the pH to 9.0. Add 30 units of dextrin per g of Geobacillus stearothermophilus (Geobacillus stearothermophilus) Tc-91 strain (Japan 〒305-8566 Tsukuba City, Ibaraki Prefecture, Higashi 1-chome, 1st place, 1st center, 6th place, independent administrative corporation product evaluation technology Base Organization Patent Biology Deposit Center (old name: Independent Administrative Legal Person Institute of Advanced Industrial Technology Patent Biology Deposit Center), deposit number FERM BP-11273, domestic deposit date: July 30, 1973, international deposit date: 2010 August 6) CGTase source, maintain pH 6.9, 50 ℃ for 18 hours to react, about 70% of hesperidin was converted to α-glycosyl hesperidin. Next, 0.05% of sodium metabisulfite was added as a...
Embodiment 2
[0264]
[0265] 4 parts by mass of 1N sodium hydroxide aqueous solution was heated to 80° C., and this temperature was maintained, 1 part by mass of hesperidin, 4 parts by mass of dextrin (DE10), and 0.06 parts by mass of sodium sulfite were sequentially added thereto, and stirred for 30 minutes. Dissolve, and immediately after neutralizing with 0.01N hydrochloric acid solution, add 20 units of Geobacillus stearothermophilus Tc-91 strain (Independent Administrative Legal Person Product Evaluation Technology Base Institute, Patent Biological Depository Center deposit number FERM) per gram of dextrin BP-11273) source of CGTase, maintain pH 6.0, 75 ° C, stirred for 24 hours. When the obtained enzyme reaction solution was sampled and analyzed by HPLC, about 69% of the hesperidin was converted into α-glycosyl hesperidin. Next, sodium metabisulfite was added as a reducing agent to the obtained enzyme reaction solution to 0.03%, and after heating at 90°C for 120 minutes, 50 units o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com