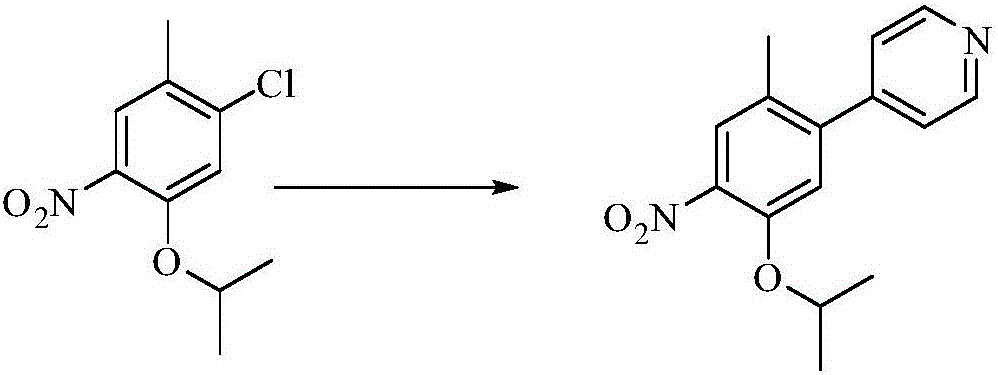Preparation method of 2-isopropoxy-5-methyl-4-(piperidine-4-yl) aniline dihydrochloride
A technology of aniline dihydrochloride and propyloxy, which is applied in the field of medicine, can solve the problems of long reaction time of the preparation method, harsh conditions of the preparation method, and low safety, and achieve short synthesis time, easy operation and control, and economical energy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
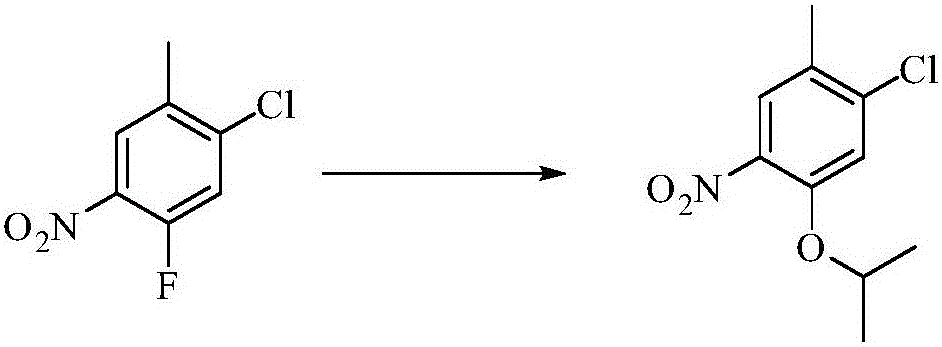
[0030] Embodiment 1: Preparation of 1-chloro-5-isopropyloxy-2-methyl-4-nitrobenzene
[0031]
[0032] Add 1.5L of isopropanol, 1-chloro-5-fluoro-2-methyl-4-nitrobenzene (260g) and add solid potassium hydroxide (116g) in batches at a temperature of 15-25°C, and keep warm for 20 ℃ reaction, monitor the disappearance of raw materials, stop stirring, add the reaction solution to 5L water, filter, and dry the filter cake to obtain the product 1-chloro-5-isopropyloxy-2-methyl-4-nitrobenzene, 271g , Yield: 86.3%.
Embodiment 2
[0033] Example 2: Preparation of 4-(5-isopropyloxy-2-methyl-4-nitrophenyl)pyridine
[0034]
[0035] 4-pyridineboronic acid (51.5g), 1-chloro-5-isopropyloxy-2-methyl-4-nitrobenzene (96.2g), 2-dicyclohexylphosphine-2',6'- Dimethoxy-biphenyl (6.87g), catalyst Pd2 (dba) 3 (7.68g), potassium phosphate (245.7g), 1.4-dioxane (840ml), water (240ml) add in the reaction flask, nitrogen Substitute three times, keep the internal temperature 60-70°C under the protection of nitrogen, monitor the disappearance of raw materials, and stop the reaction. When the temperature of the reaction liquid is lowered to below 30°C, add activated carbon, stir, spread and filter with diatomaceous earth, separate the filtrate, distill out the dioxane from the organic phase under reduced pressure, add 500ml of n-heptane, stir and crystallize at 40°C. Cool to 10°C, filter and dry to obtain the product 4-(5-isopropyloxy-2-methyl-4-nitrophenyl)pyridine, 89g, yield 77.9%.
Embodiment 3
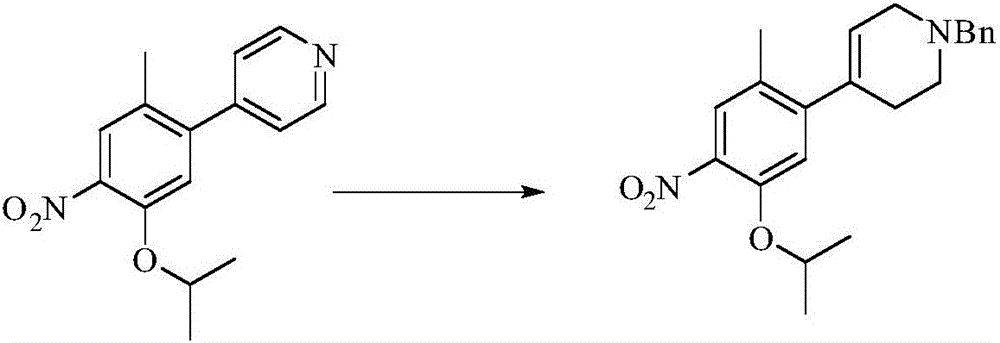
[0036] Example 3: Preparation of 1-benzyl-4-(5-isopropyloxy-2-methyl-4-nitrophenyl)-1,2,3,6-tetrahydropyridine
[0037]
[0038] 4-(5-isopropyloxy-2-methyl-4-nitrophenyl)pyridine (13.7g), benzyl bromide (11.4g) and toluene (150ml) were reacted under heating for 12 hours. Monitor the disappearance of raw materials, cool to 20-25°C, filter with suction, add the filter cake to 100ml of methanol, add lithium chloride (0.57g), control the temperature at 20-30°C, add sodium borohydride (5.1g) while stirring. After the addition, slowly rise to 20-30°C for 8 hours of reaction. After monitoring the completion of the reaction, 10 ml of saturated ammonium chloride aqueous solution was added dropwise to the reaction solution, and the methanol was distilled off under reduced pressure. Add 50ml ethyl acetate, 50ml water solution. The organic phase was dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain 1-benzyl-4-(5-isopropyloxy-2-methyl-4-nitrophenyl)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com