Phosphate mineral inhibitor as well as preparation method and use thereof
A technology of phosphorous acid, which is used in the field of phosphate mineral inhibitor-N-naphthyl-α-aminoaryl phosphonic acid compound, can solve the problems of high acid ion content, weak inhibition ability, scaling of equipment and pipelines, etc. Achieve the effect of high separation efficiency, strong inhibition and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
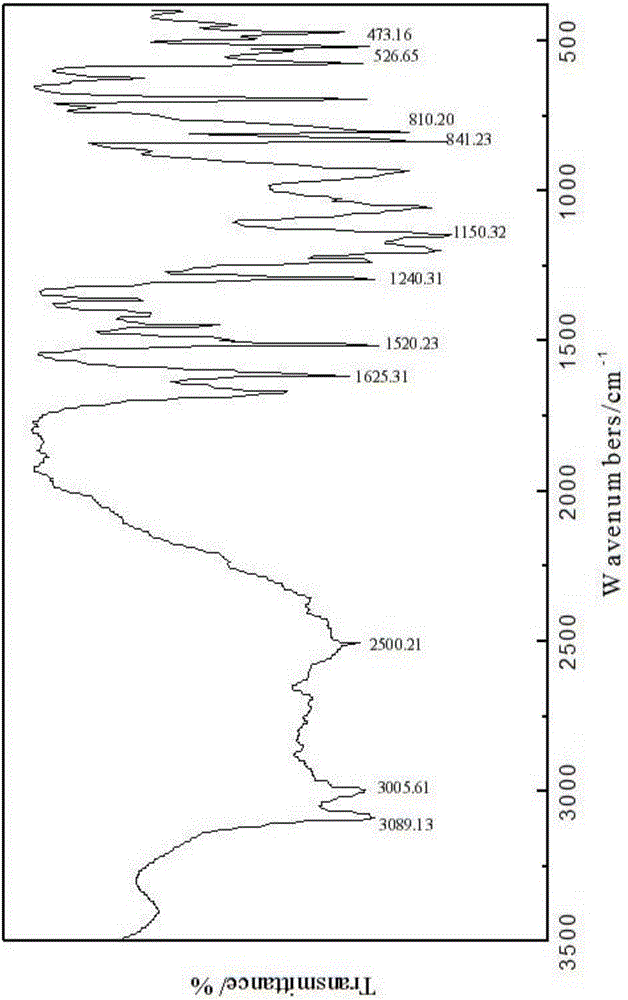
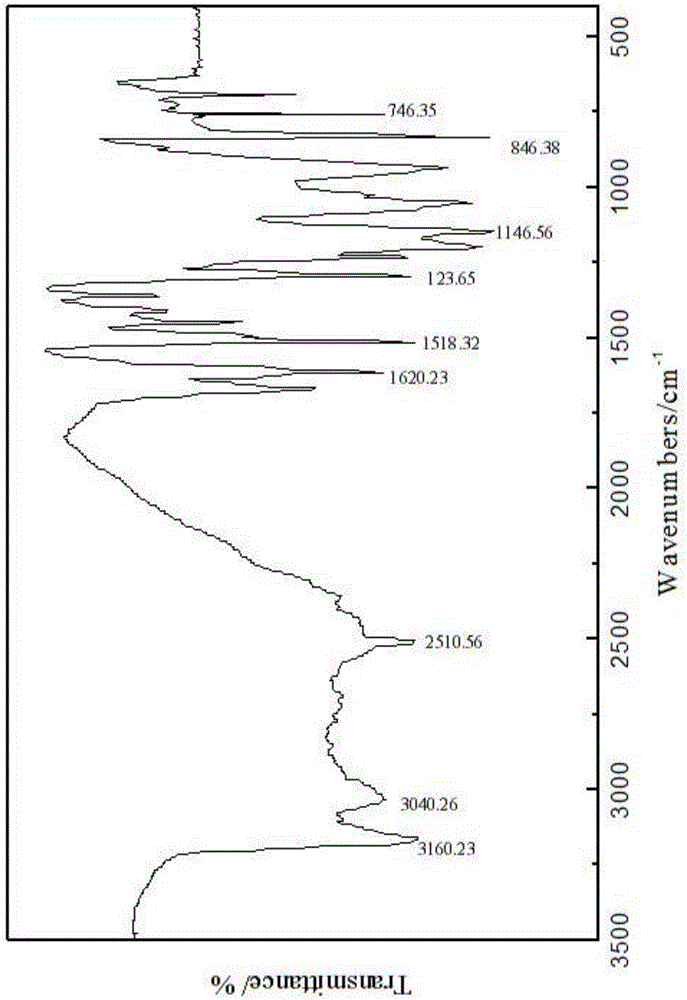
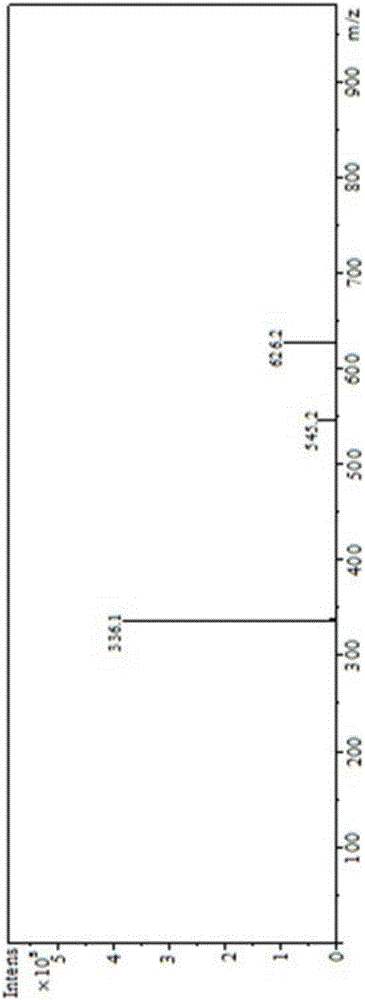
[0023] Embodiment 1, a kind of N-naphthyl-alpha-aminoaryl phosphonic acid: its general molecular formula is as formula ( ) as shown in:
[0024]
[0025] in:
[0026] R 1 is H, F, Cl, Br or C 1~4 hydrocarbon group;
[0027] R 2 It is phenyl, benzyl, phenethyl, styryl or various substituted phenyl, benzyl, phenethyl, styryl. The N-naphthyl-α-aminoaryl phosphonic acid is prepared by Mannich reaction of 1-naphthylamine, aromatic aldehyde and phosphorous acid under acidic conditions. The molar ratio of raw materials 1-naphthylamine, aromatic aldehyde, phosphorous acid and concentrated hydrochloric acid is 1:1:1:0.9.
Embodiment 2
[0028] Embodiment 2, in a kind of N-naphthyl-α-aminoaryl phosphonic acid described in embodiment 1, the mol ratio of raw material 1-naphthylamine, aromatic aldehyde, phosphorous acid, concentrated hydrochloric acid is 1: 1.4: 1.4: 1.1. All the other are identical with embodiment 1.
Embodiment 3
[0029] Embodiment 3, in a kind of N-naphthyl-α-aminoaryl phosphonic acid described in embodiment 1, the mol ratio of raw material 1-naphthylamine, aromatic aldehyde, phosphorous acid, concentrated hydrochloric acid is 1:1.1:1.1: 1. All the other are identical with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com