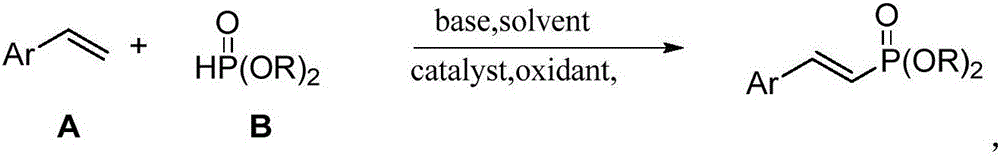Preparation method of olefin phosphate compound
A technology for alkene phosphoric acid esters and compounds is applied in the field of preparation of alkene phosphoric acid ester compounds, can solve the problems of poor control of stereoselectivity, low atom economy and the like, and achieves the effects of low price, avoiding the use of precious metals, and efficient synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Take 0.05mmol of cuprous chloride and 0.1mmol of ferric chloride, add them into the reaction tube, replace the air in the reaction tube with argon, add 0.5mmol of styrene, 2.0mmol of diethyl phosphite, and 1.0mmol of di-tert-butyl base peroxide, 0.5mmol triethylamine, 2mL dimethyl sulfoxide solvent, in an oil bath at 110°C, stop the reaction after 16 hours of reaction, filter, wash with water, remove the organic solvent by rotary evaporation, and obtain pure The yield of enophosphate is 78%.
Embodiment 2
[0019] Take 0.05mmol cuprous chloride, 0.1mmol ferric chloride, add to the reaction tube, replace the air in the reaction tube with argon, add 0.5mmol p-methylstyrene, 2.0mmol diethyl phosphite, 1.0mmol Di-tert-butyl peroxide, 0.5mmol triethylamine, 2mL dimethyl sulfoxide solvent, in an oil bath at 110°C, stop the reaction after 16 hours of reaction, filter, wash with water, remove the organic solvent by rotary evaporation, and pass through column chromatography on silica gel , to obtain the pure target product, the yields were 82%.
Embodiment 3
[0021] The procedure of this example is the same as that of Example 2, the only difference is that styrene is replaced by 2,4,6-trimethylstyrene, and the yield is 63%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com