Bimetallic phosphide hydrogen evolution catalyst and preparation method
A phosphide and catalyst technology, which is applied in the field of double metal phosphide hydrogen evolution catalyst and preparation, can solve the problems of poor stability of metal phosphide, unfavorable phosphide application, high equipment requirements, etc., achieve low raw material cost and reduce environmental hazards , The effect of the simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Weigh 2.43g CoCl 2 Place in 40mL absolute ethanol, stir at room temperature until the metal salt dissolves into a homogeneous solution A; weigh 5.62g triphenylphosphine oxide (OPPh 3 ) in 60mL of absolute ethanol, stirred at room temperature until dissolved to form a colorless and clear solution B; slowly drop solution A into solution B, and continuously stirred for 2h to obtain a precipitate, which was washed with absolute ethanol and dried to obtain Co -OPPh 3 Precursor.
[0025] Co-OPPh 3 The precursor was fully ground, and 0.5g was weighed and placed in a tube furnace. Under a nitrogen atmosphere, the temperature was raised to 600°C at 2°C / min and kept for 2h; the resulting product was repeatedly washed with absolute ethanol and dried to obtain CoP x / PC electrocatalyst (PC is phosphorus-doped carbon material).
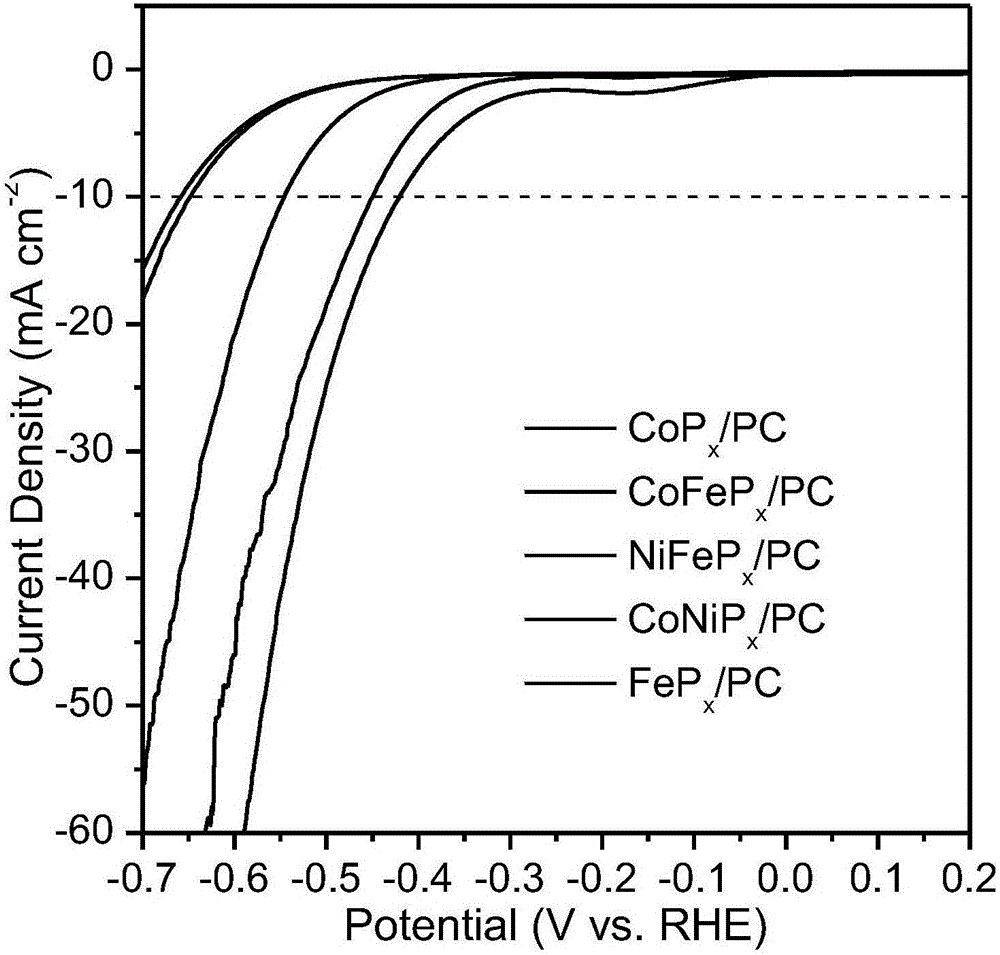
[0026] CoP prepared in this example x / PC catalyst has good dispersibility in solvent, under alkaline conditions, the current density is 10mA / cm 2 , ...
Embodiment 2
[0028] Weigh 2.73g FeCl 3 Place in 40mL absolute ethanol, stir at room temperature until the metal salt dissolves into a homogeneous solution A; weigh 5.62g triphenylphosphine oxide (OPPh 3 ) was placed in 60mL of absolute ethanol, stirred at room temperature until dissolved to form a colorless and clear solution B; solution A was slowly added dropwise into solution B, and stirred continuously for 2 hours to obtain a precipitate, which was washed with absolute ethanol and dried to obtain Fe -OPPh 3 Precursor.
[0029] Fe-OPPh 3 The precursor was fully ground, and 0.5g was weighed and placed in a tube furnace. Under a nitrogen atmosphere, the temperature was raised to 600°C at 2°C / min and kept for 2h; the obtained product was repeatedly washed with absolute ethanol and dried to obtain FeP x / PC electrocatalyst.
[0030] FeP prepared in this embodiment x / PC catalyst has good dispersibility in solvent, under alkaline conditions, the current density is 10mA / cm 2 , the hydro...
Embodiment 3
[0032] Weigh 1.21g NiCl respectively 2 and 1.37g FeCl 3 Place in 40mL absolute ethanol, stir at room temperature until the metal salt dissolves into a homogeneous solution A; weigh 5.62g triphenylphosphine oxide (OPPh 3 ) in 60 mL of absolute ethanol, stirred at room temperature until dissolved to form a colorless and clear solution B; slowly drop solution A into solution B, and continuously stirred for 2 hours to obtain a precipitate, which was washed with absolute ethanol and dried to obtain NiFe -OPPh 3 Precursor.
[0033] NiFe-OPPh 3 The precursor was fully ground, and 0.5g was weighed and placed in a tube furnace. Under a nitrogen atmosphere, the temperature was raised to 600°C at 2°C / min and kept for 2h; the resulting product was repeatedly washed with absolute ethanol and dried to obtain NiFeP x / PC electrocatalyst.
[0034] The NiFeP prepared in this embodiment x / PC catalyst has good dispersibility in solvent, under alkaline conditions, the current density is 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com