Norbornene group capping benzoxazine oligomer and preparation method thereof
An alkenyl-terminated, benzoxazine technology, applied in the direction of organic chemistry, to achieve the effect of simple preparation process, low equipment requirements, and improved processability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A preparation method of a norbornene-terminated benzoxazine oligomer, the specific steps are:
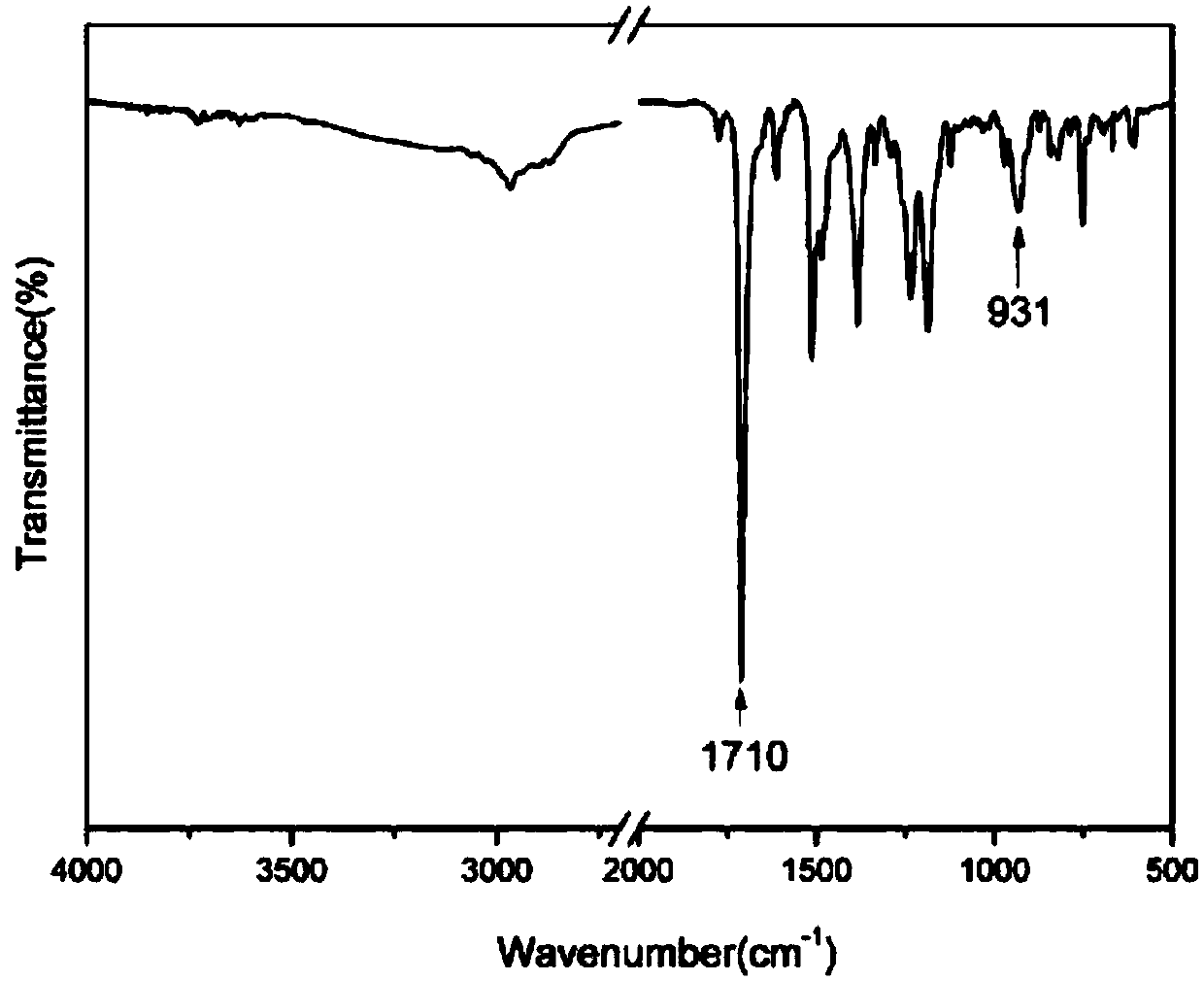
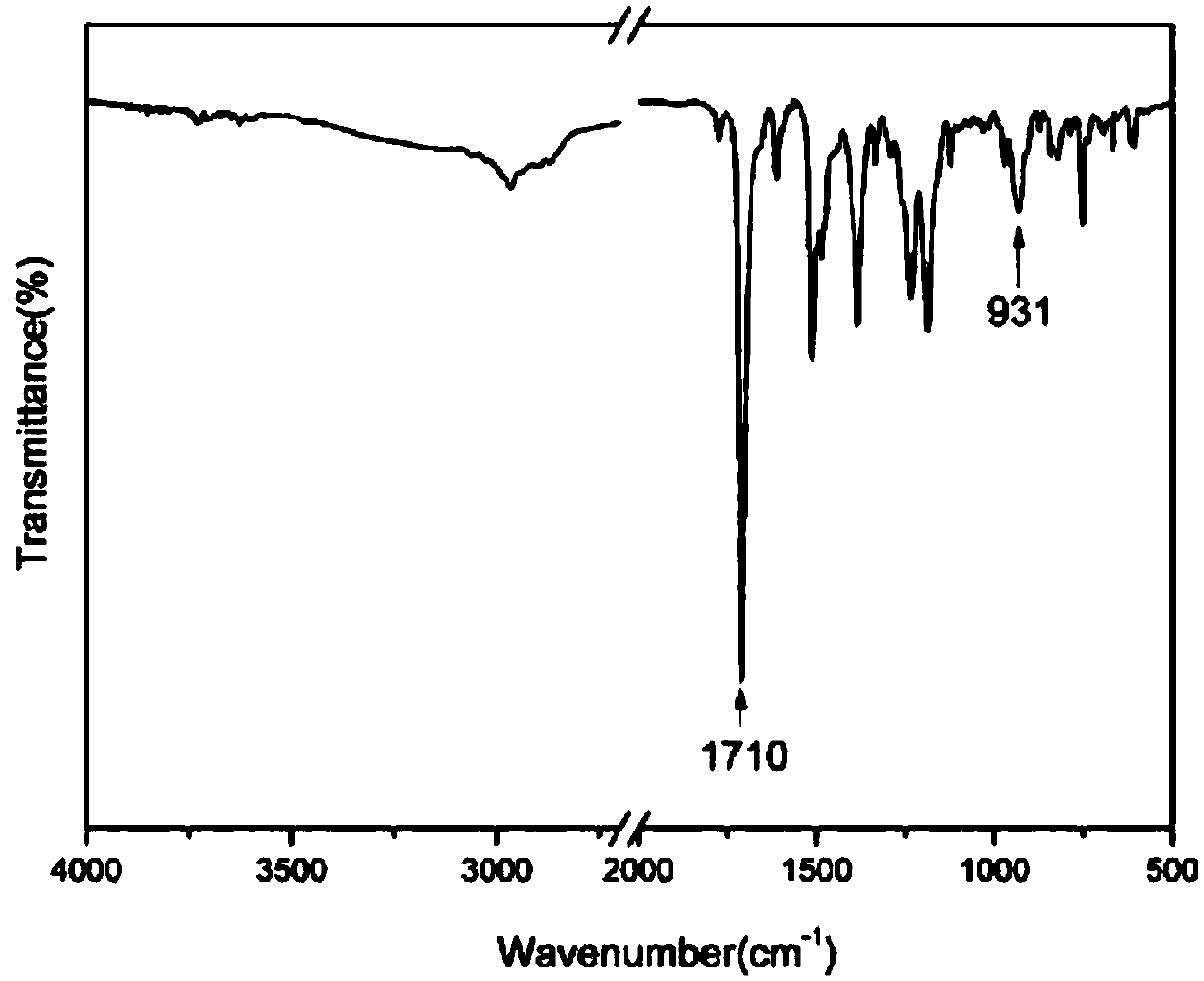
[0039] (1) 10.9g (0.1mol) of 2-aminophenol and 16.4g (0.1mol) of norbornene dianhydride are added to the reaction flask, and 30ml of glacial acetic acid is added to the flask, and the reaction system is heated from room temperature to 120 ℃, stirred with a magnetic stirrer for 6 hours, poured the reacted mixture into deionized ice water for precipitation, collected the precipitate and dried it in a vacuum electric furnace for 10-12 hours to obtain 23.4 g of white product o-norbornene phenol, yield 92%. The reaction equation is as follows:
[0040]
[0041](2) Weigh 25.5g (0.1mol) of ortho-norbornene functionalized phenol obtained in the previous step, 30.0g (0.15mol) of 4,4-dihydroxydiphenylmethane, 4,4-diaminodiphenyl Methane (DDM) 39.6g (0.2mol), paraformaldehyde 24g (0.8mol) join in the reaction flask that stirrer, thermometer and condensing tube are housed, in reacti...
Embodiment 2
[0048] The 4,4-diaminodiphenylmethane in Example 1 was replaced with 4,4-diaminodiphenyl ether, and the other steps were the same as those in Example 1.
[0049] Wherein the specific chemical structure of 4,4-diaminodiphenyl ether is:
[0050] In the second step reaction, the amount of reactant was changed to: Weigh 25.5g (0.1mol) of ortho-norbornene functionalized phenol obtained in the previous step reaction, 30.0g (0.15mol) of 4,4-dihydroxydiphenylmethane mol), diaminodiphenyl ether 40.0g (0.2mol) and paraformaldehyde 24g (0.8mol).
[0051] The structural formula of gained benzoxazine oligomer is:
[0052]
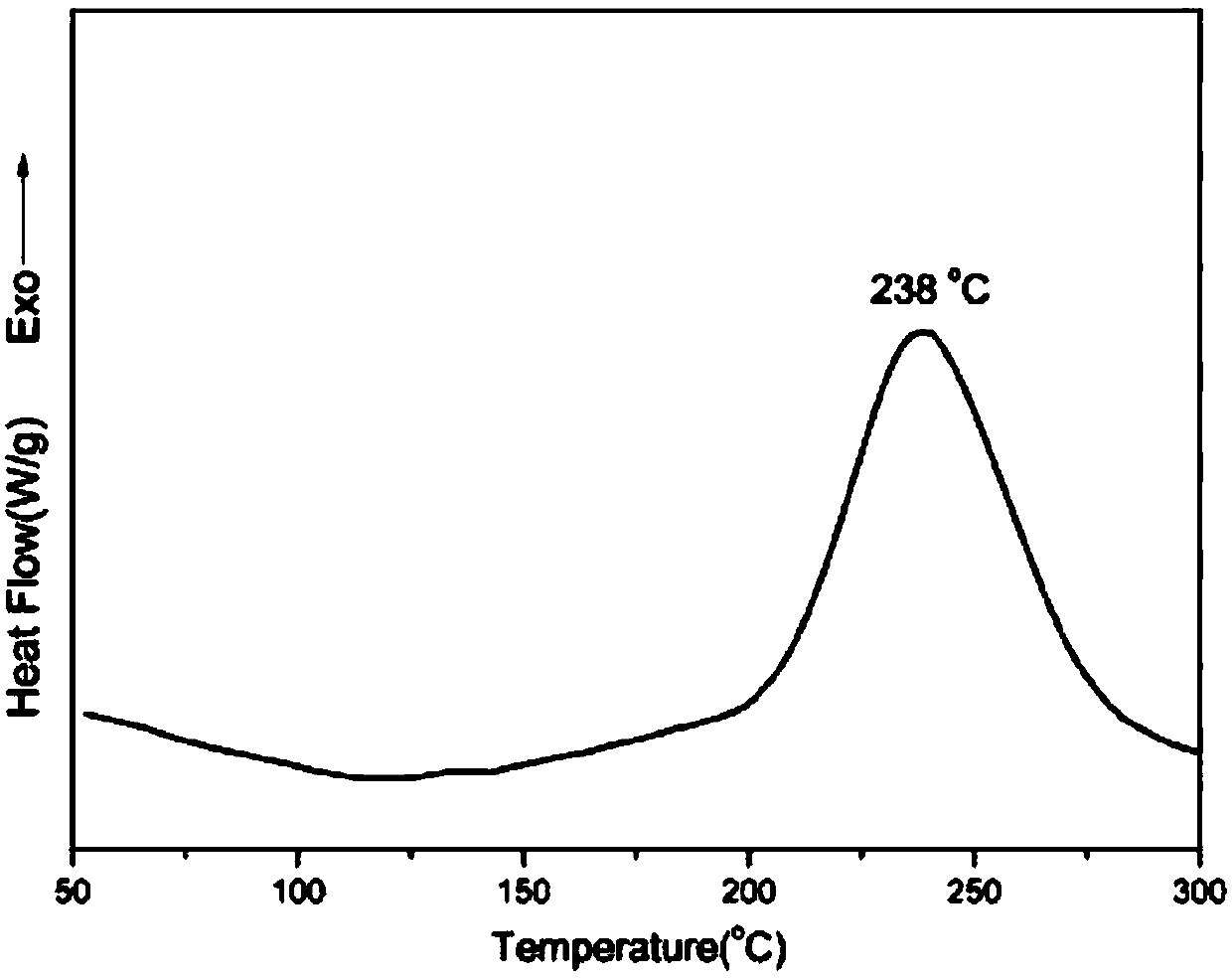
[0053] After ring-opening and solidification of the benzoxazine oligomer obtained in this example, its glass transition temperature was measured to be 402° C., its 5% thermal weight loss temperature was 533° C., and its carbon residue rate at 800° C. was 75%.
Embodiment 3
[0055] Using bisphenol A and DDM as reactants
[0056] The 4,4-dihydroxydiphenylmethane in Example 1 was replaced with bisphenol A, and other steps were the same as those in Example 1.
[0057] Wherein the specific chemical structure of bisphenol A is:
[0058] In the second step reaction, the amount of reactants was changed to: Weigh 25.5g (0.1mol) of ortho-norbornene functionalized phenol obtained in the previous step reaction, 34.2g (0.15mol) of bisphenol A, 4,4- Diaminodiphenylmethane (DDM) 39.6 g (0.2 mol) and paraformaldehyde 24 g (0.8 mol).
[0059] The structural formula of gained benzoxazine oligomer is:
[0060]
[0061] After ring-opening and solidification of the benzoxazine oligomer obtained in this example, its glass transition temperature was measured to be 385° C., its 5% thermal weight loss temperature was 517° C., and its carbon residue rate at 800° C. was 71%.
[0062] The advantage of the present invention is to introduce cross-linkable and high tem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| carbon residual rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com