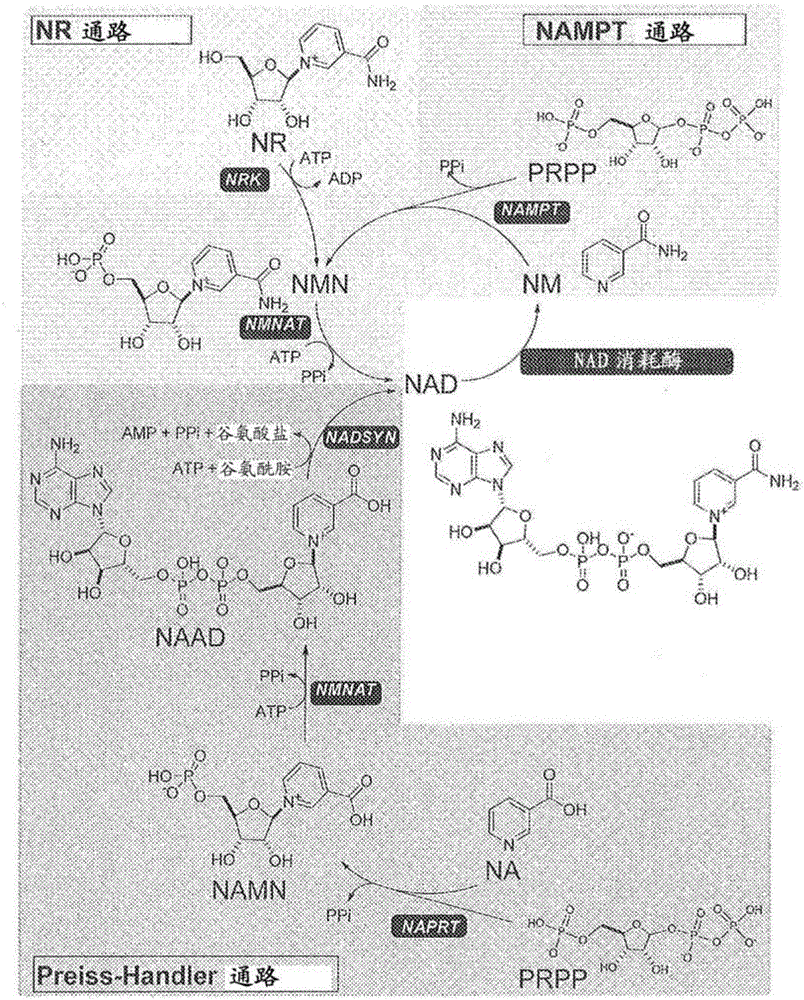
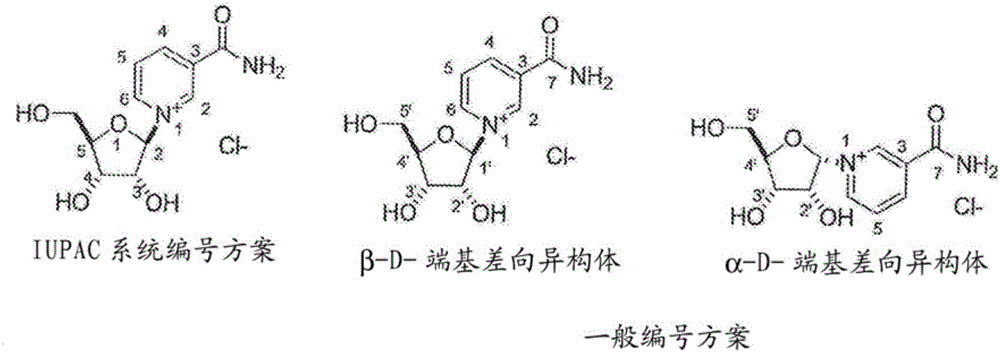
Nicotinamide riboside analogs and pharmaceutical compositions and uses thereof
A technology of nicotinamide riboside hydrogenated product and nicotinamide riboside, which is applied in the field of the composition of nicotinamide riboside analogues, and can solve the problems of bioavailability limitation, cell toxicity, side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
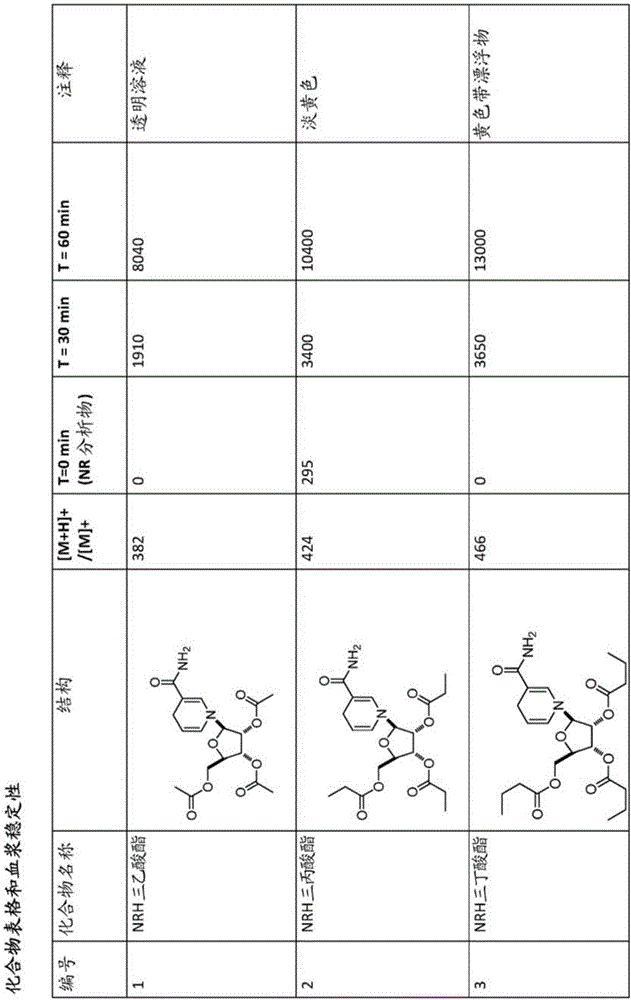
[0327] Embodiment 1: the synthesis of NRH triacetate .
[0328]
[0329] Step 1. 3-carbamoyl-1-((2R,3R,4R,5R)-3,4-diacetoxy-5-(acetoxymethyl)tetrahydrofuran-2-yl)pyridine-1- Onium triflate (NR triacetate triflate). At room temperature, to 27.98g (229mmol) nicotinamide in 350mL CH 3To the suspension in CN was added 73 mL (403 mmol) trimethylsilyl triflate (TMSOTf) in one portion. The niacinamide is completely dissolved within 5 minutes. Separately prepare α / β-D-ribofuranose-1,2,3,5-tetra-O-acetate 24.31g (76.39mmol) in 30mL CH 3 The solution in CN was then added to the nicotinamide solution in one batch. Dissolve the last trace of ribose ester in 10 mL CH 3 CN and added it to the reaction mixture. The solution was stirred at room temperature for 30 min, then dissolved by adding 1 mL of 1.2M NaHCO 3(水溶液) to quench the excess TMSOTf, followed by the addition of 20 g solid NaHCO in small batches 3 to control CO 2 release. The suspension was stirred at room temperatur...
Embodiment 2
[0332] Embodiment 2: the synthesis of NRH tripropionate
[0333]
[0334] Step 1. α / β-D-ribofuranose-1,2,3,5-tetra-O-propionate. To 1.11 g (6.76 mmol) of 1-O-methyl-α / β-D-furan Add 20mL (150mmol) propionic anhydride and 1.0mL (13mmol) propionic acid to ribose. The mixture was stirred and heated at 100°C for 1.5 hours, then it was stored at -20°C overnight (18h). In the morning, the reaction mixture was warmed to 25 °C, then 0.2 mL of H 2 SO 4 , and the reaction mixture was stirred at room temperature for 2 hours. Pour this solution into 100 mL of cold 1.2M NaHCO 3 solution, and the mixture was washed with CH 2 Cl 2 (2x 100 mL) extraction. CH that will be merged 2 Cl 2 Layer with 1.2M NaHCO 3(水溶液) (1x 100 mL) and brine (1x 100 mL), filtering if necessary to break up any emulsion. The organic layer was washed with Na 2 SO 4 Dry, filter, and concentrate in vacuo. The residue was purified by silica gel chromatography (40 g column) with 40 mL of pentane, a gradie...
Embodiment 3
[0339] Embodiment 3: the synthesis of NRH tri-n-butyrate
[0340]
[0341] Step 1. α / β-D-ribofuranose-1,2,3,5-tetra-O-n-butyrate. Add 1.00 g (6.09 mmol) of 1-O-methyl-α / β-D- Add 20mL (120mmol) butyric anhydride and 1.0mL (11mmol) butyric acid to ribofuranose. The stirred reaction mixture was heated at 100 °C for 1.5 h, then cooled to room temperature. Then add 0.30 mL (5.6 mmol) of 98% H 2 SO 4 , and the reaction was stirred at room temperature for 1 h. LCMS (also known as LC-MS or liquid chromatography-mass spectrometry) showed that the reaction was complete after this time. Pour this solution into 100 mL of cold 1.2M NaHCO 3 solution, and the mixture was washed with CH 2 Cl 2 (2x 100 mL) extraction. CH that will be merged 2 Cl 2 layer with 1.2M NaHCO 3(水溶液) (1x 100 mL) and brine (1x 100 mL), filtering if necessary to break up any emulsion. The organic layer was washed with Na 2 SO 4 Dry, filter, and concentrate in vacuo. The residue was purified by silica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com