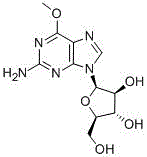
Antitumor drug nelarabine powder-injection composition
An anti-tumor drug, the technology of nelarabine powder, which is applied in the field of medicine, can solve the problems of differences in bioavailability, etc., and achieve the effects of small differences in loading, significant drug efficacy, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Preparation of Nelarabine Crystals
[0036] (1) Add 10g of crude nelarabine to 110ml of ethanol and acetonitrile (1:1) mixture, heat to 75°C and stir for 1 hour;
[0037] (2) When the crude nelarabine is completely dissolved, add 0.02g activated carbon, continue to heat to reflux, keep for 20 minutes, filter while hot, and concentrate the filtrate;
[0038] (3) Add 330ml isopropanol under stirring, and let it cool to room temperature;
[0039] (4) Continue to stand for 2 hours, cool to -10°C to crystallize in an ice bath, grow crystals for 8 hours, filter, wash the crystals with a small amount of absolute ethanol cooled to 0°C at least once, and dry under reduced pressure (the drying temperature is controlled at 50 The temperature is below ℃, the pressure is controlled at 10mmHg, and the drying time is 12 hours) to obtain 9.875 g of Nelarabine crystal compound with a yield of 98.75% and a purity of 99.99%.
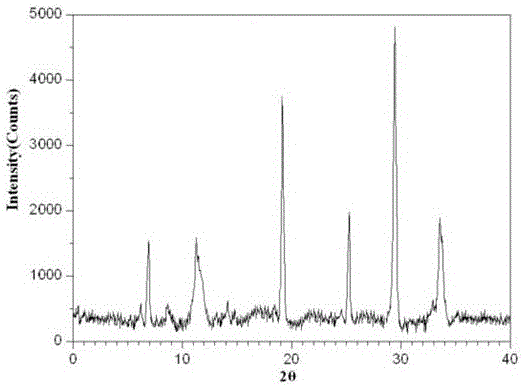
[0040] The X-ray powder diffraction pattern of the prepared ...
Embodiment 2
[0043] Example 2: Preparation of Nelarabine Crystals
[0044] (1) Add 10g of crude nelarabine to 105ml of ethanol and acetonitrile (1:1) mixture, heat to 78°C and stir for 0.8 hours;
[0045] (2) When the crude nelarabine is completely dissolved, add 0.025g of activated carbon, continue to heat to reflux, keep for 15 minutes, filter while hot, and concentrate the filtrate;
[0046] (3) Add 420ml isopropanol under stirring, and let it cool to room temperature;
[0047] (4) Continue to stand for 2.5 hours, cool down to -15°C to crystallize in an ice bath, grow crystals for 7 hours, filter, wash the crystals with a small amount of absolute ethanol cooled to 0°C at least once, and dry under reduced pressure (the drying temperature is controlled at 50 The temperature is below ℃, the pressure is controlled at 12mmHg, and the drying time is 13 hours) to obtain 9.856g of Nelarabine crystal compound with a yield of 98.56% and a purity of 99.99%.
[0048] The X-ray powder diffraction pattern of ...
Embodiment 3
[0049] Example 3: Preparation of Nelarabine Crystals
[0050] (1) Add 10g of crude nelarabine to 115ml of ethanol and acetonitrile (1:1) mixture, heat to 72°C and stir for 1.2 hours;
[0051] (2) When the crude nelarabine is completely dissolved, add 0.015 g of activated carbon, continue to heat to reflux, keep for 25 minutes, filter while hot, and concentrate the filtrate;
[0052] (3) Add 230ml of isopropanol under stirring, let it cool to room temperature;
[0053] (4) Continue to stand for 1.5 hours, cool down to -5°C to crystallize in an ice bath, grow crystals for 9 hours, filter, wash the crystals with a small amount of absolute ethanol cooled to 0°C at least once, and dry under reduced pressure (the drying temperature is controlled at 50 The temperature is below ℃, the pressure is controlled at 8mmHg, and the drying time is 11 hours) to obtain 9.849g of Nelarabine crystal compound, with a yield of 98.49% and a purity of 99.99%.
[0054] The X-ray powder diffraction pattern of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com