Method for constructing engineering arterial blood vessel in vivo by taking melt-spinning fiber as skeleton
A melt spinning, arterial blood vessel technology, applied in the direction of prosthesis, single-component polyester rayon, single-component copolyester rayon, etc. The effect of good mechanical strength, good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
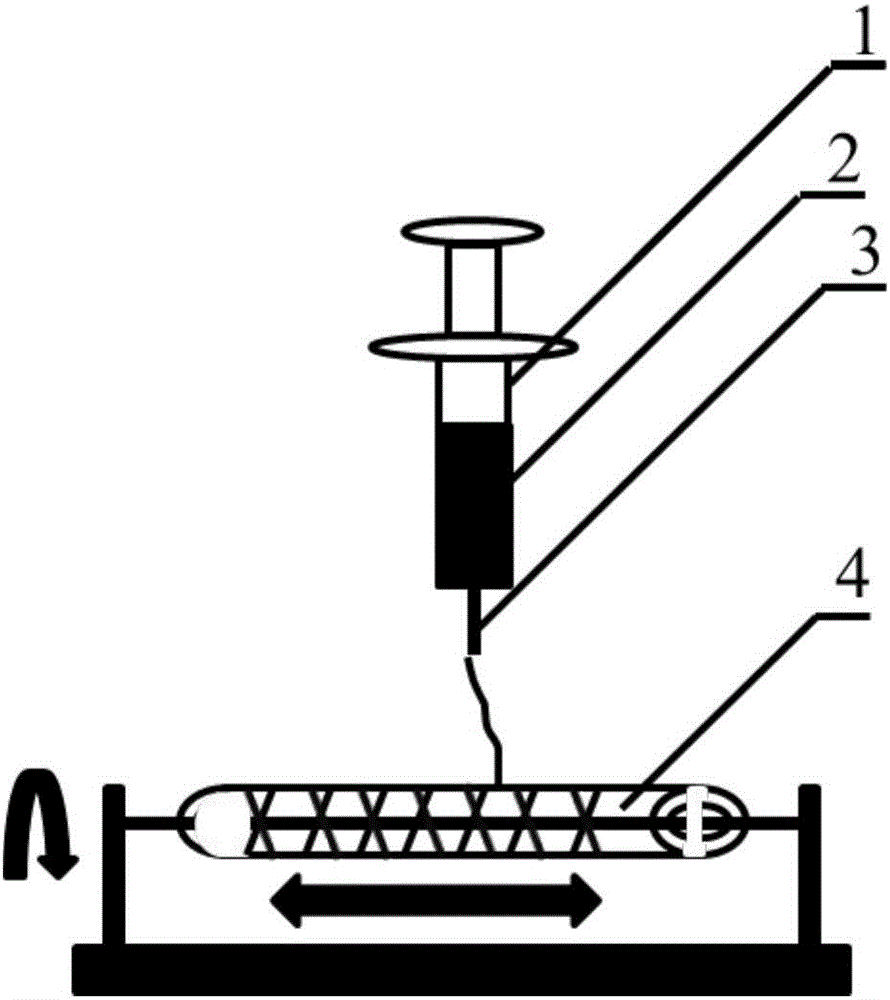
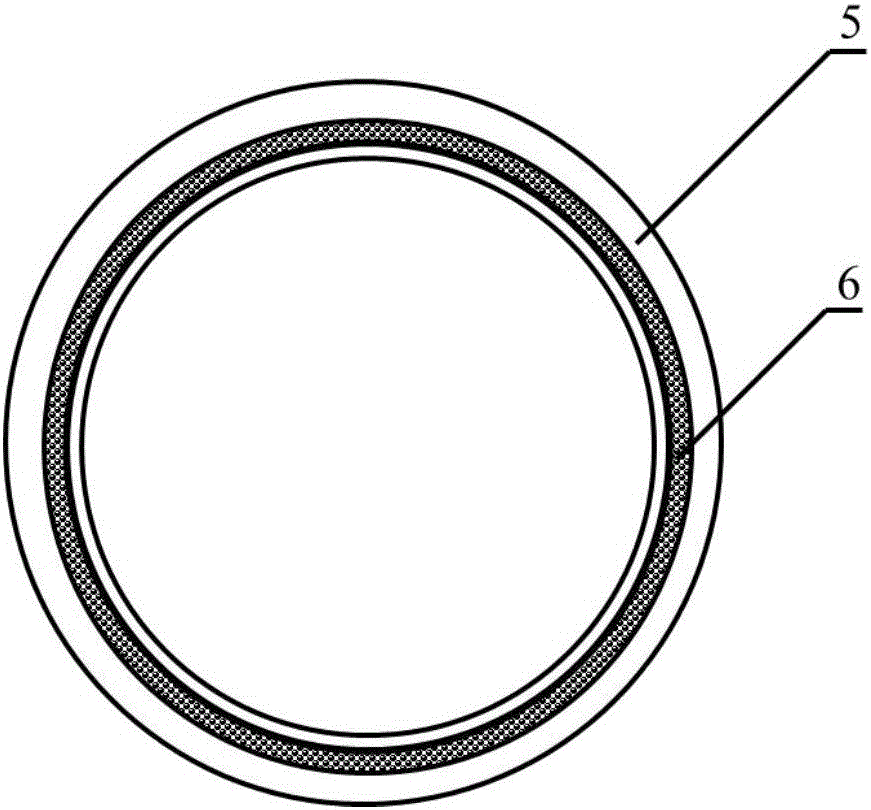
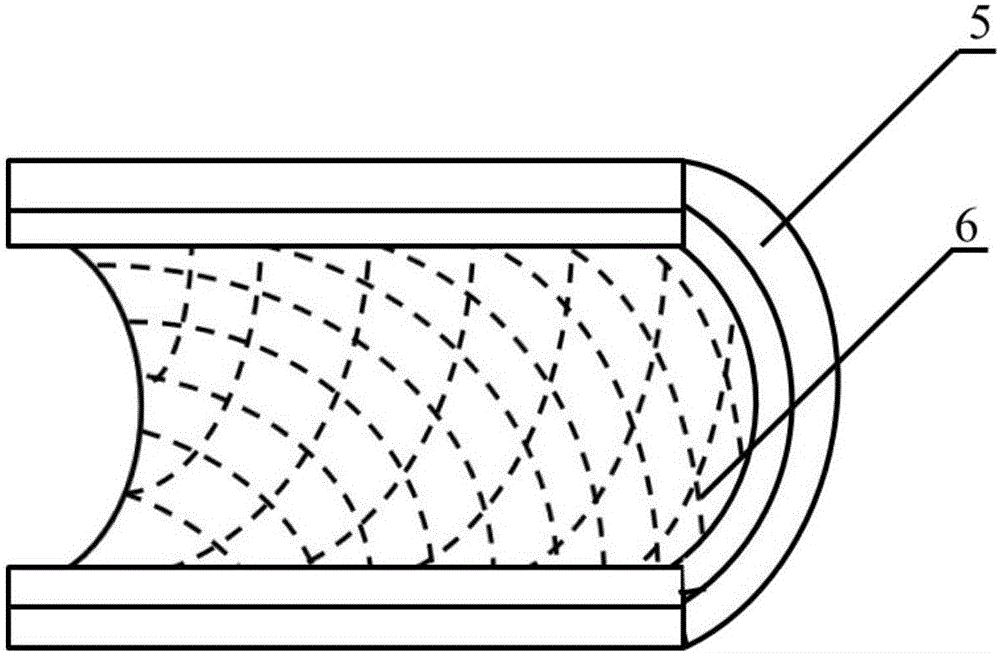
[0023] A method for constructing engineered arteries in vivo using melt-spun fibers as a skeleton, using polycaprolactone (PCL) spun fibers as a skeleton, and subcutaneous rats as a bioreactor for the preparation of engineered arteries in vivo, specific steps as follows:
[0024] 1. Preparation of PCL melt-spun fiber and medical silicone tube composite mandrel
[0025]Weigh 5.0g, with a number average molecular weight of 80000PCL, place it in a closed stainless steel syringe wrapped in a fuser, and heat it at 220°C for 1h. Put a medical silicone tube with an outer diameter of 2mm and an inner diameter of 1mm on a stainless steel rod with a diameter of 1mm and connect it to the rotating motor. The speed is set to 300r / min, and the translation speed is set to 10mm / s. The spinning time was set to 10 min, and the composite mandrel of PCL melt-spun fiber and medical silicone tube with a fiber angle of 45° was prepared. After the spinning is completed, the silicone tube is withdr...
Embodiment 2
[0031] A method for constructing engineered arteries in vivo with melt-spun fibers as a skeleton, using polycaprolactone (PCL) spun fibers as a skeleton, and preparing an in vivo engineered arteries in a rabbit subcutaneous bioreactor, the specific steps are as follows :
[0032] 1. Preparation of PCL melt-spun fiber and medical silicone tube composite mandrel
[0033] Weigh 5.0g, number average molecular weight of 80000PCL, put it in a closed stainless steel syringe wrapped in a fuser, and heat at 220°C for 1h. Put a medical silicone tube with an outer diameter of 2mm and an inner diameter of 1mm on a stainless steel rod with a diameter of 1mm and connect it to the rotating motor. The speed is set to 300r / min, and the translation speed is set to 10mm / s. The spinning time was set to 15 min, and the composite mandrel of PCL melt-spun fiber and medical silicone tube with a fiber angle of 45° was prepared. After the spinning is completed, the silicone tube is withdrawn from th...
Embodiment 3
[0039] A method for constructing engineered arteries in vivo with melt-spun fibers as a skeleton, using polyglycolic acid (PGA) spun fibers as a skeleton, and subcutaneous rats as a bioreactor for the preparation of engineered arteries in vivo, the specific steps are as follows :
[0040] 1. Preparation of PGA melt-spun fiber and medical silicone tube composite mandrel
[0041] Weigh 5.0 g of PGA with a number average molecular weight of 100,000, place it in a closed stainless steel syringe wrapped in a fuser, and heat at 300°C for 1 hour. Put a medical silicone tube with an outer diameter of 2mm and an inner diameter of 1mm on a stainless steel rod with a diameter of 1mm and connect it to the rotating motor. The speed is set to 300r / min, and the translation speed is set to 10mm / s. The spinning time was set to 10 min, and the composite mandrel of PGA melt-spun fiber and medical silicone tube with a fiber angle of 45° was prepared. After the spinning is completed, the silico...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com