Preparation method of shape-controlled silver sulfide
A silver sulfide and morphology technology, applied in chemical instruments and methods, silver compounds, silver compounds, etc., can solve problems such as the inability to adjust the optical and photoelectric properties of silver sulfide, the inability to effectively control the morphology and particle size of silver sulfide, etc. Achieve the effect of low cost, little pollution and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A preparation method of silver sulfide of the present invention, comprises the following steps:
[0039] (1) Prepare silver nitrate solution, polyvinylpyrrolidone solution and cystine solution respectively earlier, then these three kinds of solutions are mixed, control silver nitrate concentration in the mixed solution to be 0.003mol / L, the pH value of solution is 10, polyethylene The amount of pyrrolidone added is the same as that of silver nitrate, and the ratio of cystine to silver nitrate is 1:1;
[0040] (2) Transfer the mixed solution of step (1) to a 50mL autoclave with a polytetrafluoroethylene liner, and react at 180°C for 10 hours;
[0041] (3) After the reaction product after step (2) is naturally cooled to room temperature, it is centrifuged, washed with deionized water and ethanol for 3 times, and finally vacuum-dried for 8 hours to obtain a silver sulfide product.
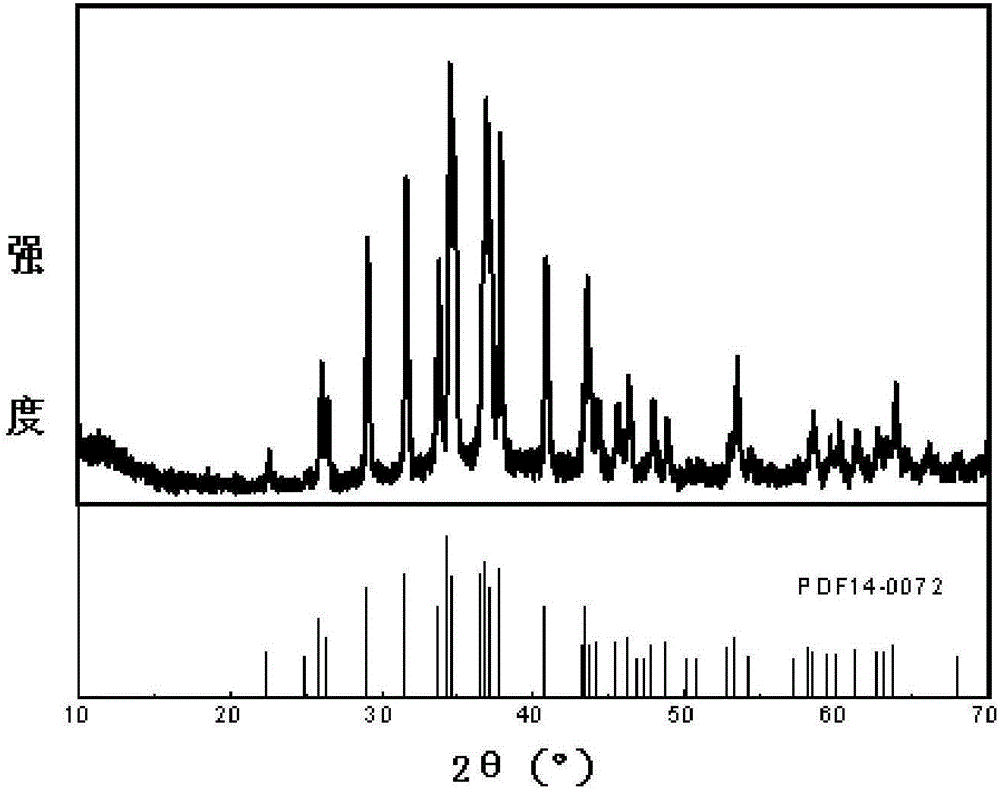
[0042] Test the X-ray diffraction pattern of the silver sulfide product gained in the pres...
Embodiment 2
[0044] A preparation method of silver sulfide of the present invention, comprises the following steps:
[0045] (1) First prepare silver ammonia solution (formed by adding silver nitrate to ammonia water), polyethylene glycol solution and cysteine solution respectively, and mix the three solutions, and control the concentration of silver ammonia in the mixed solution to be 0.005 mol / L, the pH value of the solution is 9, the polyethylene glycol addition is equal to the quality of the silver ammonia solution, and the ratio of the amount of cysteine to the silver ammonia substance is 1.2:1;
[0046] (2) Transfer the mixed solution of step (1) to a 50mL autoclave with a polytetrafluoroethylene liner, and react at 160°C for 8 hours;
[0047] (3) The reaction product after step (2) was naturally cooled to room temperature, centrifuged, washed three times with deionized water and ethanol respectively, and finally vacuum-dried for 8 hours to obtain a silver sulfide product.
[00...
Embodiment 3
[0050] A preparation method of silver sulfide of the present invention, comprises the following steps:
[0051] (1) Silver nitrate solution, polyethylene glycol solution and methionine solution were first prepared respectively, then these three solutions were mixed, and the concentration of silver nitrate in the mixed solution was controlled to be 0.01mol / L, and the pH value of the solution was 11, polyethylene glycol The amount of alcohol added is 0.25 times the mass of silver nitrate, and the ratio of the amount of methionine to silver nitrate is 0.8:1;
[0052] (2) Transfer the mixed solution of step (1) to a 50mL autoclave with a polytetrafluoroethylene liner, and react at 120°C for 12 hours;
[0053] (3) After the reaction product after step (2) is naturally cooled to room temperature, it is centrifuged, washed with deionized water and ethanol for 3 times, and finally vacuum-dried for 8 hours to obtain a silver sulfide product.
[0054] Test the SEM picture of the silver...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com