Preparation method of alpha-Fe2O3 magnetic nano-powder material
A magnetic nanometer and powder material technology, applied in the direction of iron oxide/iron hydroxide, iron oxide, etc., can solve the problems of high condition control requirements, high product cost, long preparation cycle, etc., and achieves low equipment requirements and low cost. , the effect of high practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: α-Fe 2 o 3 Preparation of magnetic nanopowder materials
[0022] Prepare FeCl with a concentration of 0.8 mol / L 3 Solution, accurately transfer 50 mL to a 250 mL Erlenmeyer flask, according to urea and Fe 3+ Accurately weigh urea with a molar ratio of 10:1 and add it into a 250 mL Erlenmeyer flask, stir to dissolve, heat the solution to 85°C, and stir for 4 h. After centrifugation, the precipitate was washed 5 times with distilled water. After centrifugation, the precipitate was placed in a drying oven, and the precursor was obtained after drying. Grind the precursor, put it into a temperature-programmed resistance furnace for calcination at 600 °C for 4 h, and cool naturally to obtain α-Fe 2 o 3 Magnetic nano powder material.
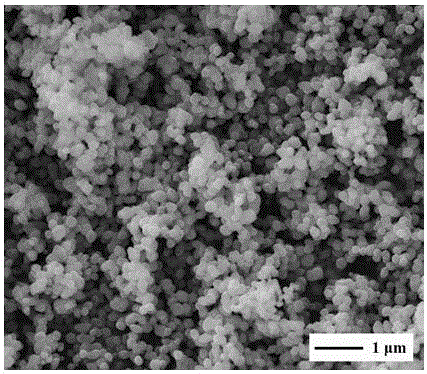
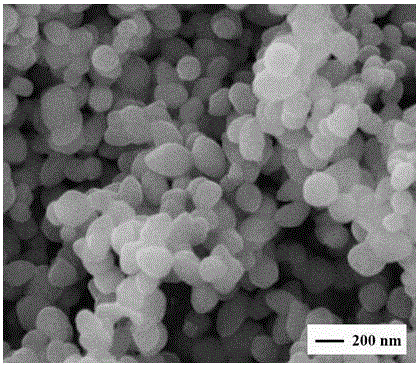
[0023] figure 1 α-Fe prepared for this example 2 o 3 The panoramic view of the scanning electron microscope picture of the magnetic nano-powder material. From the panoramic view, it can be seen that the prepared iron oxide na...
Embodiment 2
[0027] Example 2: α-Fe 2 o 3 Preparation of magnetic nanopowder materials
[0028] Fe(NO 3 ) 3 Solution, accurately transfer 50 mL to a 250 mL Erlenmeyer flask, according to urea and Fe 3+ Accurately weigh urea with a molar ratio of 5:1 and add it into a 250 mL Erlenmeyer flask, stir to dissolve, raise the temperature of the solution to 90°C, and stir for 1 h. After centrifugation, the precipitate was washed 5 times with distilled water. After centrifugation, the precipitate was placed in a drying oven, and the precursor was obtained after drying. The precursor was ground, put into a temperature-programmed resistance furnace for calcination at 500 °C for 2 h, and cooled naturally to obtain α-Fe 2 o 3 Magnetic nano powder material, its saturation magnetization is 0.35 emu / g.
Embodiment 3
[0029] Example 3: α-Fe 2 o 3 Preparation of magnetic nanopowder materials
[0030] Prepare FeCl with a concentration of 0.5 mol / L 3 Solution, accurately transfer 50 mL to a 250 mL Erlenmeyer flask, according to urea and Fe 3+Accurately weigh urea with a molar ratio of 8:1 and add it into a 250 mL Erlenmeyer flask, stir to dissolve, raise the temperature of the solution to 98°C, and stir for 6 h. After centrifugation, the precipitate was washed 5 times with distilled water. After centrifugation, the precipitate was placed in a drying oven, and the precursor was obtained after drying. Grind the precursor, put it into a temperature-programmed resistance furnace for calcination at 700 °C for 3 h, and cool naturally to obtain α-Fe 2 o 3 Magnetic nano powder material, its saturation magnetization is 0.41 emu / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Saturation magnetization | aaaaa | aaaaa |
| Saturation magnetization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com