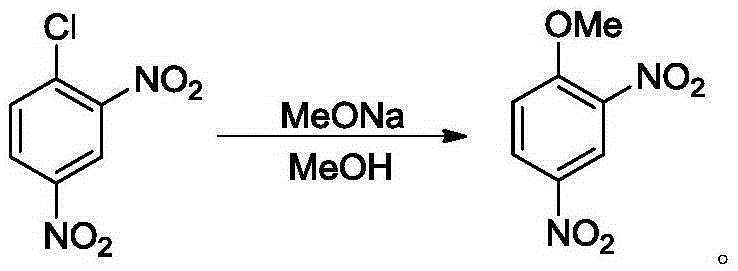
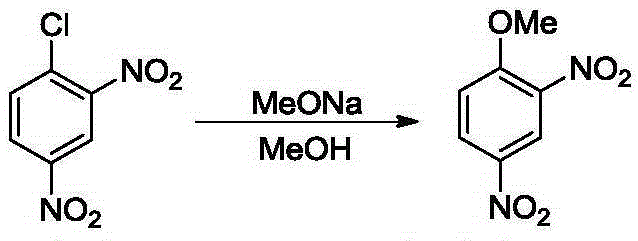
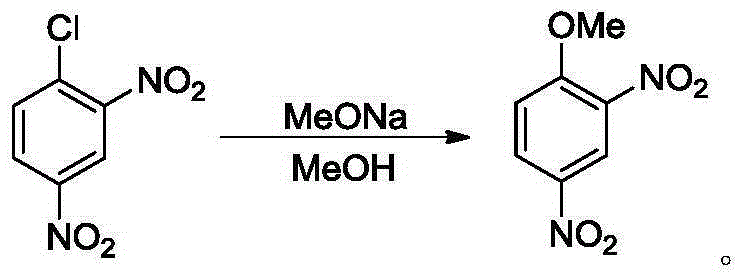
2,4-dinitroanisole synthesis method
A technology of dinitroanisole and synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as troublesome post-processing, long reaction time, large amount of reaction solvent, etc., and achieve the goal of reaction Mild conditions, no side reactions, high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] Add 2,4-dinitrochlorobenzene (5.0g, 25.0mmol) and methanol (5mL) into a 100mL two-necked reaction flask, raise the temperature to an internal temperature of 40°C (feeding temperature), stir until the solids are completely dissolved, and add 4.7g 30% by mass concentration of sodium methoxide in methanol solution (containing 1.4 g of sodium methoxide, 1.02 equiv., 25.5 mmol), dripped in 20 minutes. After dropping, the temperature was raised to an internal temperature of 60°C (reaction temperature), and the reaction was carried out for 7 hours until the pH value of the reaction solution was 8-9, and the reaction was stopped. Filtration, the filter cake was washed with water 4 times (4x5mL), the solid was collected and vacuum-dried to obtain light yellow solid 2,4-dinitroanisole (4.69g, yield 95.8%), detected by HPLC, the product purity was greater than 99% %.
Embodiment 2
[0026] The reaction steps and operations are the same as in Example 1, except that the molar ratio of 2,4-dinitrochlorobenzene to sodium methoxide in the reaction is 1:1. The reaction was stopped, and 2,4-dinitroanisole (4.63 g, yield 94.5%) was obtained after post-processing. The purity of the product was greater than 99% as detected by HPLC.
Embodiment 3
[0028] The reaction steps and operations are the same as in Example 1, except that the molar ratio of 2,4-dinitrochlorobenzene to sodium methoxide in the reaction is 1:2. The reaction was stopped, and 2,4-dinitroanisole (4.20 g, yield 85.7%) was obtained after post-processing. The purity of the product was greater than 99% as detected by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com