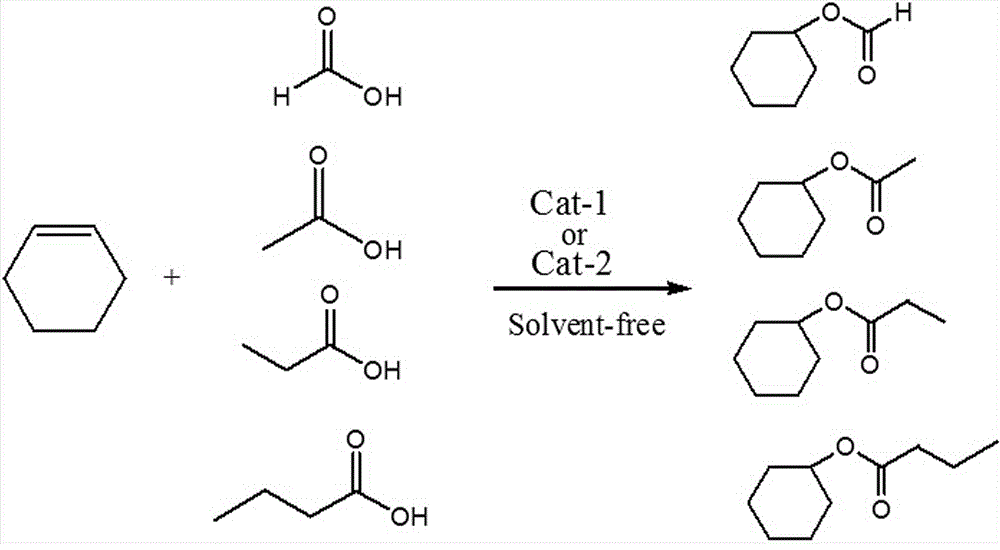
Polysulfo-functionalized heteropolyacid ionic hybrid with multiple heteropolyacid negative ions as well as preparation method and application thereof
A heteropolyacid anion, polysulfonic acid functional technology, applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of easy deactivation, low catalytic activity, and complicated catalyst preparation process. , to achieve good amphipathic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
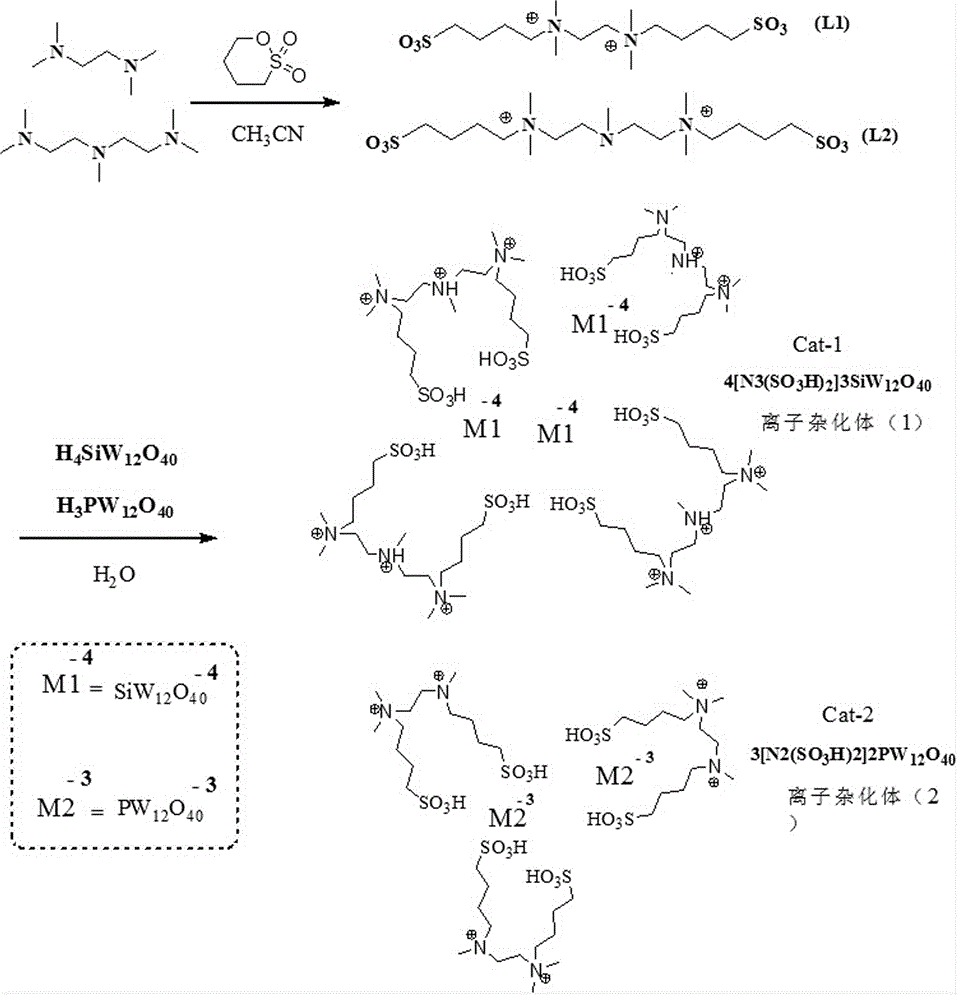
[0050] ionic hybrid 4[N 3 (SO 3 h) 2 ]3SiW 12 o 40 Synthesis
[0051] Step S101: Add 9.5 g of 1,4-butane sultone and 20 ml of acetonitrile in sequence in a reaction flask equipped with stirring, stir at room temperature for 15 minutes, add 6.0 g of pentamethyldiethylenetriamine dropwise, and heat up after the addition Reaction at 45°C for 24 h; the crude product was vacuum-dried at 75°C for 8 hours after suction filtration and diethyl ether rinse to obtain 14.5 g of the intermediate amphoteric salt L2 with a yield of 93.7%;
[0052] L2 is an off-white solid, yield 96%, m.p. 58 °C. 1 HNMR (D 2 O, 400 MHz,), δ : 2.16~2.25(m, 8H, 2CH 2 ); 2.26(s, 3H, CH 3 ); 2.934~2.97(m, 8H, 4CH 2 ); 3.13 (s, 12H, 4CH 3 ); 3.47~3.52(m, 8H, 4CH 2 ). 13 CNMR (D 2 O, 100 MHz), δ : 32.13 (CH 2 CH 2 N), 34.42 (CH 2 CH 2 CH 2 N), 43.35 (NCH 3 ), 48.14 (CH 2 SO 3 ), 51.85 ((NCH 3 ), 57.64 (NCH 2 ), 63.67 (N + CH 2 ), 64.24 (CH 2 N + ).
[0053] Step S102: After fully di...
Embodiment 2
[0056] Ionic hybrid 3[N 2 (SO 3 h) 2 ]2PW 12 o 40 Synthesis
[0057] S101: Add 4.8g of 1,4-butane sultone and 20ml of acetonitrile in turn to a reaction flask equipped with stirring, stir at room temperature for 15 minutes, add 2.0g of tetramethylethylenediamine dropwise, and raise the temperature to 50°C after adding Reacted for 24 h; the crude product was suction-filtered, rinsed with ether, and vacuum-dried at 75°C for 8 hours to obtain 5.8 g of the intermediate amphoteric salt L1 with a yield of 86.7%;
[0058] L1 is an off-white solid in 95% yield, m.p. 66 °C. 1 HNMR (D 2 O, 400 MHz), δ : 2.26~2.47 (m, 8H, 4CH 2 ); 2.99~3.03 (t, 4H, 2CH 2 ); 3.27(s, 12H); 3.59~3.64 (t, 4H, 2CH 2 ); 3.98(t,4H,2CH 2 ). 13 CNMR (D 2 O, 100 MHz), δ : 31.85 (CH 2 CH 2 N), 35.36 (CH 2 CH 2 CH 2 N), 47.86 (CH 2 SO 3 ), 50.81 (NCH 3 ), 63.33 (NCH 2 ), 64.17 (CH 2 N).
[0059] S102: After fully dissolving 1.0 g of amphoteric salt in 15 ml of deionized water, slowly add d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com