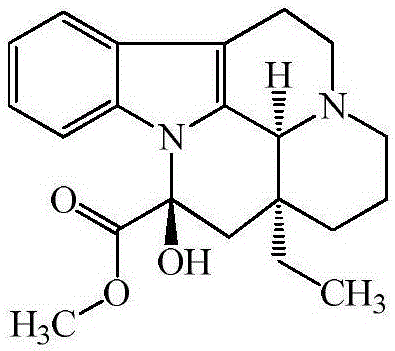
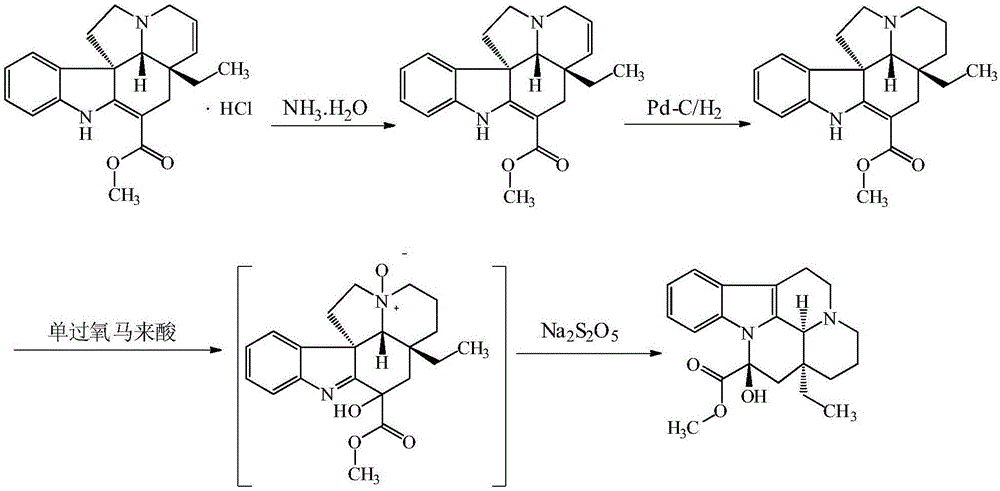
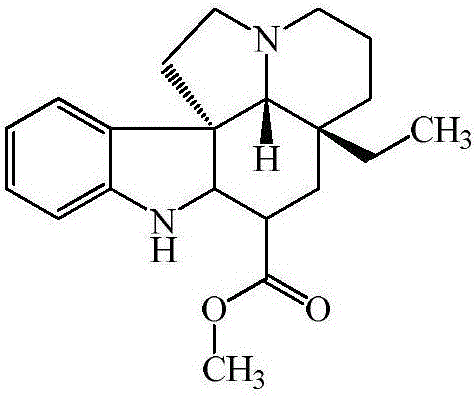
Vincamine preparation method
A vincamine and a certain amount of technology, applied in the field of compound preparation, can solve the problems of large amount of catalytic hydrogenation catalyst, high oxidant risk, and difficulty in obtaining raw materials, and achieve the effects of improved yield, easy recovery and application, and easy purchase.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The impact of using different hydrogenation reaction solvents on the reaction:
[0046] (1) Preparation of Itbonin:
[0047]Add 30.0g (80.4mmol) of bonin hydrochloride and 325.0g of petroleum ether into a 1000mL four-necked bottle, add 12.3g (87.7mmol) of ammonia water dropwise under stirring, stir at 25°C for 0.5h after dropping, and then add 330g of saturated sodium bicarbonate solution was stirred for 0.5h. After the reaction was completed, the layers were separated, and the aqueous layer was extracted twice with 2×65g petroleum ether. The organic phases were combined, dried by adding 5 g of anhydrous magnesium sulfate for 2 h, filtered with suction, and the petroleum ether was distilled off under reduced pressure at 50° C. to obtain 27.0 g of Tabonin with a mass yield of 90%.
[0048] (2) Preparation of Vinca Manfermin
[0049] Add 26.0g (77.3mmol) tabonin in the 1000ml four-necked bottle that is furnished with tee, 208.0g organic solvent dissolves, and described...
Embodiment 2
[0057] The impact of adopting different hydrogenation reaction temperatures on the reaction:
[0058] (1) Preparation of Itbonin:
[0059] Add 30.0g (80.4mmol) of bonin hydrochloride and 325.0g of petroleum ether into a 1000mL four-neck bottle, add 12.3g (87.7mmol) of ammonia water dropwise under stirring, stir at room temperature for 0.5h after dropping, and then add 330g Saturated sodium bicarbonate solution was stirred for 0.5h. After the reaction was completed, the layers were separated, and the aqueous layer was extracted twice with 2×65g petroleum ether. The organic phases were combined, dried by adding 5 g of anhydrous magnesium sulfate for 2 h, filtered with suction, and the petroleum ether was distilled off under reduced pressure at 50° C. to obtain 27.0 g of Tabonin with a mass yield of 90%.
[0060] (2) Preparation of Vinca Manfermin
[0061] Add 26.0g (77.3mmol) Tabonin to a 1000ml four-necked bottle equipped with a tee, dissolve in 208.0g methanol, add 2.6g Pd / ...
Embodiment 3
[0069] The influence of different oxidation reaction oxidant additions on the reaction:
[0070] (1) Preparation of Itbonin:
[0071] Add 30.0g (80.4mmol) of bonin hydrochloride and 325.0g of petroleum ether into a 1000mL four-neck bottle, add 12.3g (87.7mmol) of ammonia water dropwise under stirring, stir at room temperature for 0.5h after dropping, and then add 330g Saturated sodium bicarbonate solution was stirred for 0.5h. After the reaction was completed, the layers were separated, and the aqueous layer was extracted twice with 2×65g petroleum ether. The organic phases were combined, dried by adding 5 g of anhydrous magnesium sulfate for 2 h, filtered with suction, and the petroleum ether was distilled off under reduced pressure at 50° C. to obtain 27.0 g of Tabonin with a mass yield of 90%.
[0072] (2) Preparation of Vinca Manfermin
[0073] Add 26.0g (77.3mmol) Tabonin to a 1000ml four-necked bottle equipped with a tee, dissolve in 208.0g methanol, add 2.6g Pd / C und...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com