Novel fucoidan oligosaccharides as well as preparation method and application thereof
A technology of fucoidan sulfate and oligosaccharides, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of limited fucose residue quantity and types, small application range, etc. , to achieve the effects of enriching varieties, expanding the scope of use and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
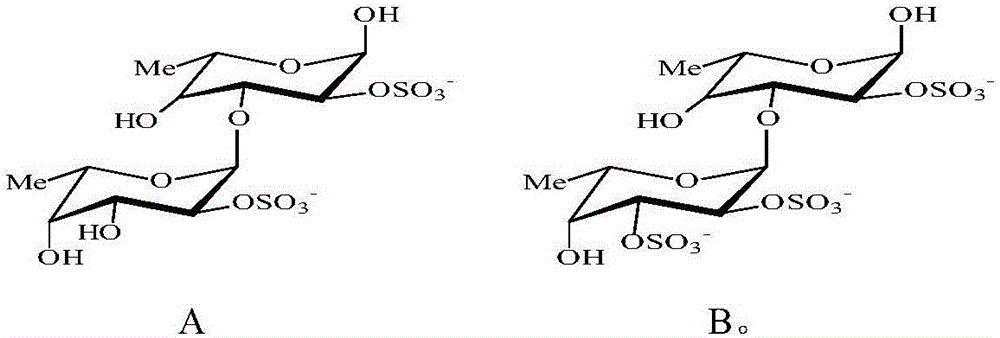
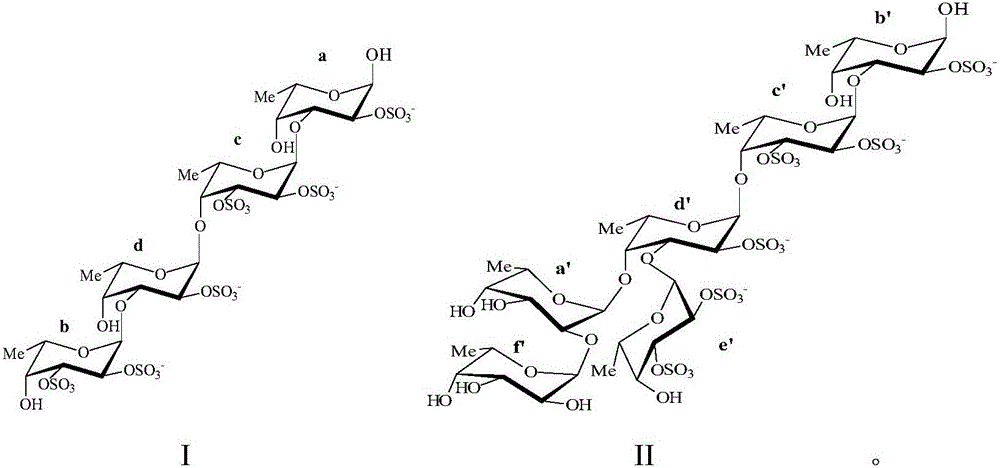
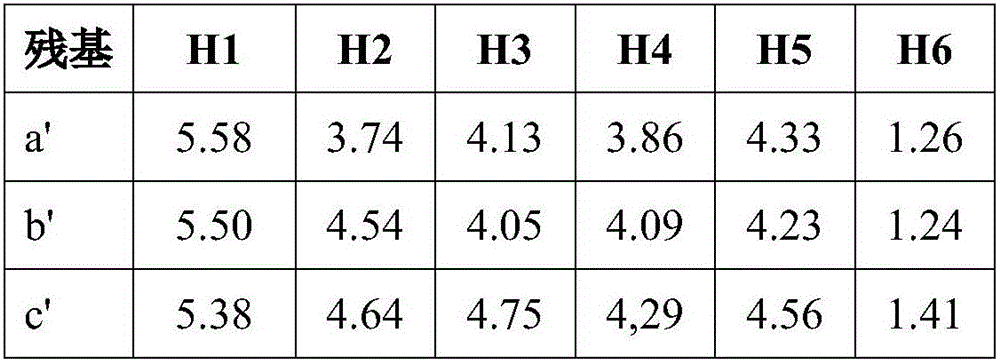
[0036] Put the recombinant fucoidanase FFA1 (1mg) into the Tris-HCl buffer solution with a pH value of 7.2, mix it with 5% copper algae (Sargassum horneri) fucoidan solution, and put the mixture at a temperature of 37°C Artificially cultivated for 72 hours, and then heated to 100°C for 5 minutes. The resulting precipitate was separated by centrifugation and discarded. Add ethanol to the supernatant until its concentration reaches 75%. The formed precipitate (polymer component) was centrifuged at 9000 g for 20 min. The supernatant (low molecular components) was poured into a column containing Q-Sepharose adsorbent, and equilibrated with pure water. The oligosaccharides were then eluted with a linear gradient of ammonium bicarbonate at a rate of 1 mL / min. First operate to obtain sulfated fucosylated oligosaccharides with molecular formula II, then operate to obtain sulfated fucosylated oligosaccharides with molecular formula I, and then freeze-dry the obtained product to obta...
Embodiment 2
[0038] Put the recombinant fucoidanase FFA1 (1mg) into the Tris-HCl buffer solution with a pH value of 7.2, mix it with 10% copper algae (Sargassum horneri) fucoidan solution, and put the mixture at a temperature of 37°C Artificially cultivated for 75 hours, and then heated to 100°C for 10 minutes. The resulting precipitate was separated by centrifugation and discarded. Add acetone to the supernatant until its concentration reaches 75%. The formed precipitate (polymer component) was centrifuged at 10000 g for 30 min. The supernatant (low molecular components) was poured into a column containing Q-Sepharose adsorbent, and equilibrated with pure water. The oligosaccharides were then eluted with a linear gradient of ammonium bicarbonate at a rate of 1 mL / min. First operate to obtain the fucoidan sulfate oligosaccharide with molecular formula II, then operate to obtain the fucoidan sulfate oligosaccharide with molecular formula I, then freeze-dry the obtained product, and obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com