Preparation method of L-phenylalanine print hydrogel based on zwitter-ion monomer
A zwitterion, phenylalanine technology, applied in chemical instruments and methods, alkali metal oxides/hydroxides, inorganic chemistry, etc., to achieve the effect of overcoming the decrease in sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The preparation method of the L-phenylalanine imprinted hydrogel based on the zwitterionic monomer of the present invention specifically comprises the following steps:
[0027] Step 1: Alkylation reaction of 1-vinylimidazole and 1,3-propane sultone to prepare zwitterionic monomer VSPIM (VSPIM is 1-vinyl-3-sulfonic acid propylimidazole);
[0028] The specific process of step 1 is:
[0029] Step 1.1, dissolve 1-vinylimidazole in acetonitrile, then add 1,3-propane sultone and react at 40-70°C for 6-24 hours to obtain a white precipitate, the mixture of 1-vinylimidazole and acetonitrile The molar ratio is 1:3~6, the molar ratio of 1-vinylimidazole to 1,3-propane sultone is 1~1:2;
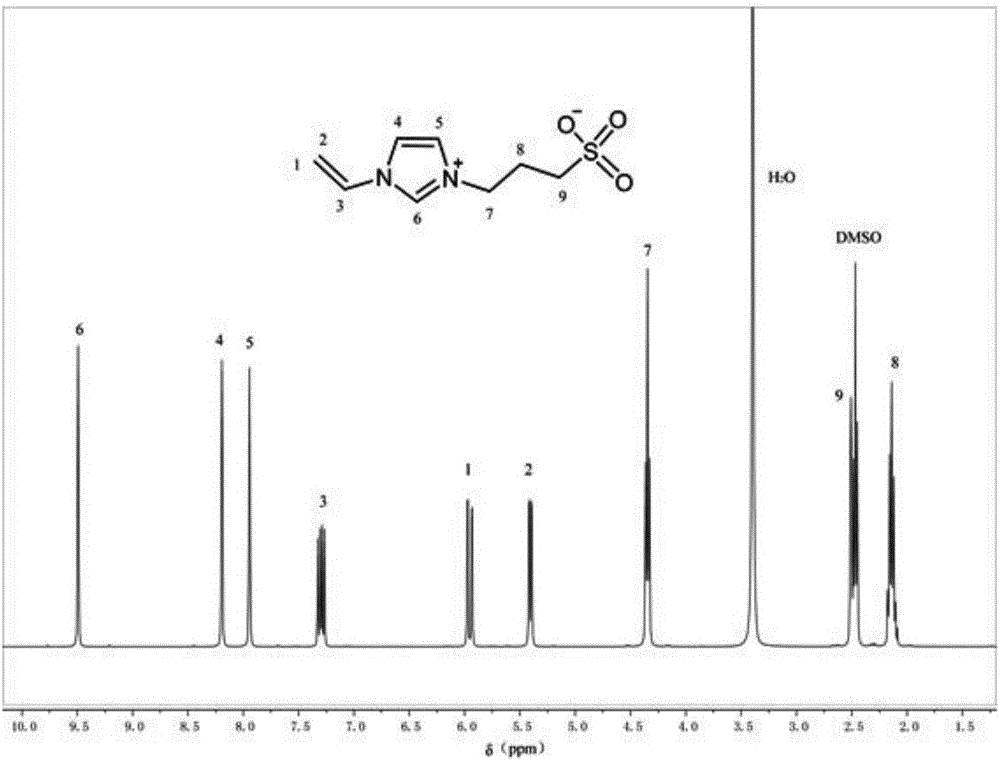
[0030] Step 1.2, wash the white precipitate obtained in step 1.1 with acetone (acetone is analytically pure acetone), and then dry it in a vacuum drying oven for 12 to 48 hours. The temperature of the drying oven is 30 to 50°C to obtain the zwitterionic monomer VSPIM , see figure 1 ;
[0031]...
Embodiment 1
[0037]Dissolve 0.1 mol of 1-vinylimidazole in 0.3 mol of acetonitrile, then add 0.1 mol of 1,3-propane sultone, and react at 40°C for 6 hours to obtain a white precipitate, the white precipitate Washing with acetone, followed by drying in a vacuum oven at 30°C for 12 hours, yielded the zwitterionic monomer VSPIM with a yield of 71.3%;
[0038] Will 1×10 -3 mol template molecule L-phenylalanine with 1×10 -3 mol of functional monomer VSPIM was dissolved in 0.3mol of water to obtain solution A, and 0.5×10 -3 mol of N,N-methylenebisacrylamide and 1.5×10 -5 mol of ammonium persulfate, after fully dissolving, pass nitrogen into the solution to remove oxygen for 5 minutes, then quickly add 1.5×10 -5 mol of tetramethylethylenediamine, the solution was reacted at 15°C for 3 hours to obtain a hydrogel, the hydrogel was washed by 0.1mol / L NaCl solution at 15°C, and the elution was measured with a UV spectrophotometer The characteristic peak at 259nm of the eluent, the washing process...
Embodiment 2
[0044] Dissolve 0.1 mol of 1-vinylimidazole in 0.5 mol of acetonitrile, then add 0.15 mol of 1,3-propane sultone, and react at 50°C for 15 hours to obtain a white precipitate, the white precipitate Washing with acetone, followed by drying in a vacuum oven at 40°C for 30 hours, yielded the zwitterionic monomer VSPIM with a yield of 86.5%;
[0045] Will 1×10 -3 mol template molecule L-phenylalanine with 5×10 -3 mol of functional monomer VSPIM was dissolved in 0.5 mol of water to obtain solution A, and 1×10 -3 mol of N,N-methylenebisacrylamide and 1.2×10 -4 mol of ammonium persulfate, after fully dissolving, pass nitrogen to the solution to remove oxygen for 15 minutes, then quickly add 1.2×10 -4 mol of tetramethylethylenediamine, the solution was reacted at 25°C for 15 hours to obtain a hydrogel, and the hydrogel was washed with a 0.3mol / L NaCl solution at 25°C, and the elution was measured with a UV spectrophotometer The characteristic peak at 259nm of the eluent, the washi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com