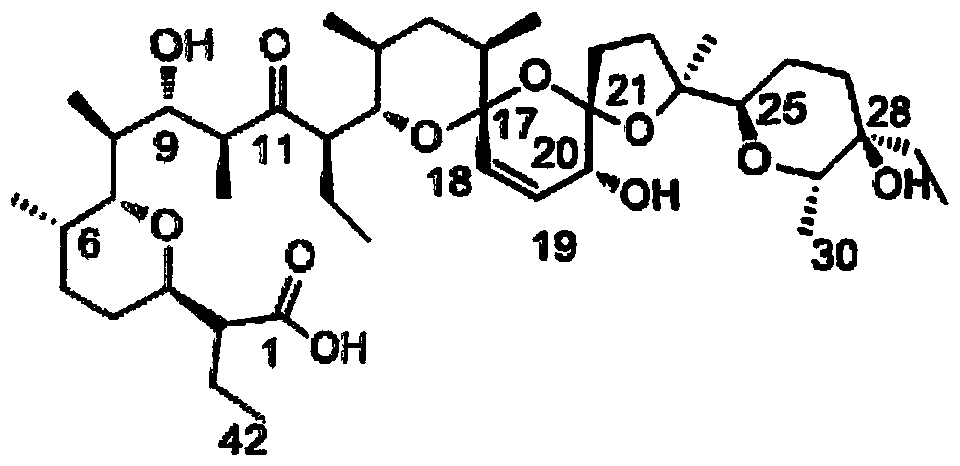
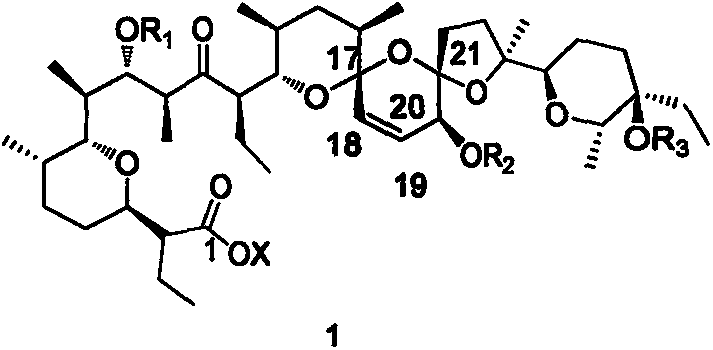
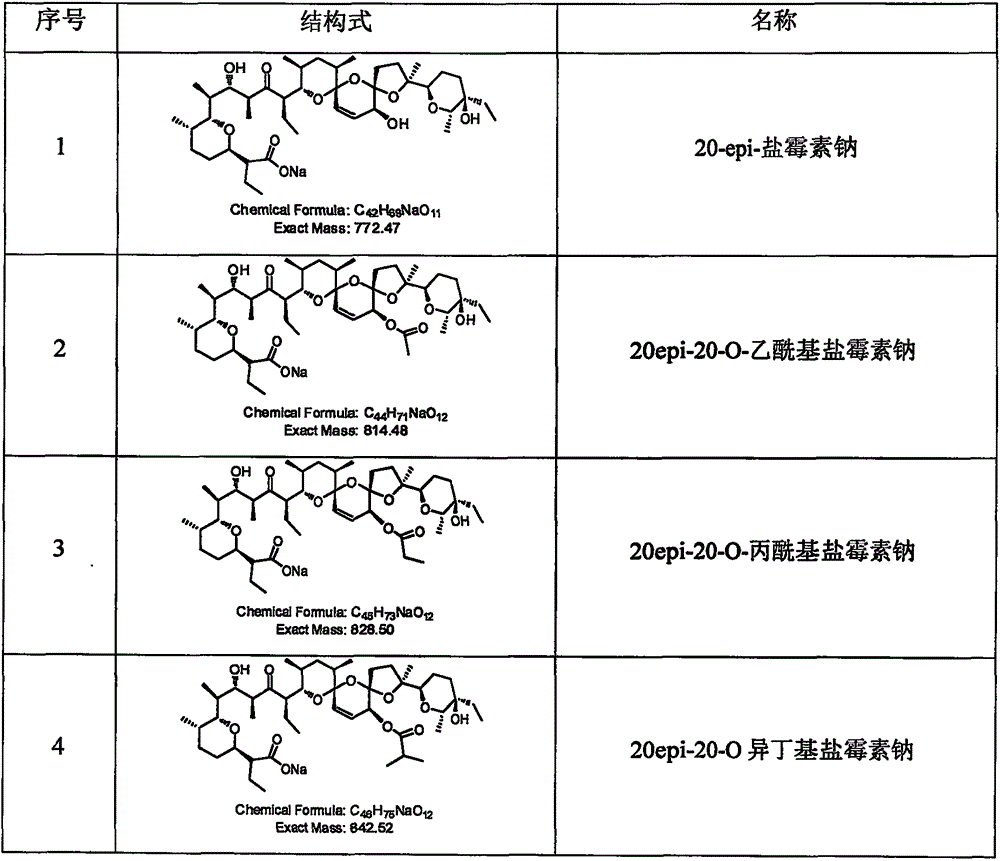
C20 site epimerization salinomycin and derivatives thereof, and preparation method and use thereof
A technology of epimerization and salinomycin, applied in organic chemistry, drug combination, pharmaceutical formulation, etc., can solve the problems of in-depth research on activity testing and unclear structure-activity relationship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0058] The preparation of preparation example 1 salinomycin trimethylsilyl ethyl ester (intermediate 1)
[0059]
[0060] Dissolve 2.4g (3.2mmol) salinomycin in 20ml dichloromethane, add 3ml (20.8mmol) trimethylsilyl ethanol, 1.8ml (10.1mmol) diisopropylethylamine (DIPEA) and 1.0g ( 3.67 mmol) N, N, N', N'-tetramethylchloroformamidine hexafluorophosphate (TCFH), stirred at room temperature for 24 h. Wash with 10ml of 0.1M aqueous HCl solution, 10ml of saturated aqueous sodium bicarbonate solution and 10ml of water respectively, combine the organic phases, dry, concentrate, and separate by column chromatography (eluent petroleum ether:ethyl acetate=3:1) to obtain the target The product was 1.36g, which was a colorless oil, and the yield was 50%. The unreacted salinomycin was recovered.
[0061] 1 H NMR (400MHz, CDCl 3 )δ6.06(d, J=10.8Hz, 1H), 5.97(d, J=10.8Hz, 1H), 4.41(dtd, J=35.3, 11.2, 5.9Hz, 2H), 4.09-3.94(m, 3H ), 3.93-3.77(m, 2H), 3.68(dd, J=10.4, 7.2Hz, 2H), 3.55(...
preparation example 220
[0062] Preparation 2 20-epi-20-O-p-nitrobenzoyl salinomycin trimethylsilyl ethyl ester (intermediate 2)
[0063]
[0064] Dissolve 1.0 g of salinomycin trimethylsilyl ethyl ester in 30 ml of tetrahydrofuran, add 3.0 g of triphenylphosphine and 0.5 g of p-nitrobenzoic acid while stirring, then add 2 ml of diisopropyl azodicarboxylate (DIAD ) was slowly added dropwise, stirred at room temperature for 5 h, and TLC detected that the reaction of the raw materials was complete. The solvent was evaporated to dryness and separated by column chromatography (petroleum ether: ethyl acetate = 8:1) to obtain 0.93 g of the target product as a colorless oil with a yield of 80%.
[0065] 1 H NMR (400MHz, CDCl 3 )δ8.27(d, J=8.2Hz, 2H), 8.16(d, J=8.2Hz, 2H), 6.51(d, J=10.6Hz, 1H), 6.33(dd, J=10.6, 5.3Hz, 1H), 5.22(d, J=5.3Hz, 1H), 4.55-4.32(m, 1H), 4.12-3.99(m, 1H), 3.76(d, J=6.5Hz, 1H), 3.69(d, J =9.6Hz, 1H), 3.59(d, J=10.3Hz, 1H), 3.49(d, J=7.5Hz, 1H), 3.24(dd, J=15.1, 7.7Hz, 1H), 3.01...
preparation example 320
[0066] Preparation Example 3 Preparation of 20-epi-salinomycin trimethylsilyl ethyl ester (intermediate 3)
[0067]
[0068] Dissolve 1.0 g of 20-epi-O-p-nitrobenzoyl salinomycin trimethylsilylethyl ester in 20 ml of methanol, add 210 mg of potassium carbonate, stir at room temperature for 30 minutes, and TLC detects that the reaction of the raw materials is complete. Evaporate the solvent to dryness, add 100ml of dichloromethane to dissolve the residue, wash the organic phase with 20ml of 0.1M NaOH aqueous solution and 20ml of water, dry, evaporate the solvent to obtain the crude product of C20-epi-salinomycin trimethylsilyl ethyl ester, pass through the column Chromatographic purification afforded 0.6g.
[0069] 1 H NMR (400MHz, CDCl 3 )δ6.06(d, J=10.8Hz, 1H), 5.97(d, J=10.8Hz, 1H), 4.41(dtd, J=35.3, 11.2, 5.9Hz, 2H), 4.09-3.94(m, 3H ), 3.93-3.77(m, 2H), 3.68(dd, J=10.4, 7.2Hz, 2H), 3.55(dd, J=10.4, 2.2Hz, 1H), 3.17(td, J=14.7, 7.3Hz, 1H), 3.08-2.91(m, 2H), 2.70(d, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com