Chimeric antigen receptor (CAR) and application thereof
A chimeric antigen receptor and antibody technology, applied in the direction of hybrid peptide, receptor/cell surface antigen/cell surface determinant, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve Issues such as increased labor and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1: Construction of Chimeric Antigen Receptor
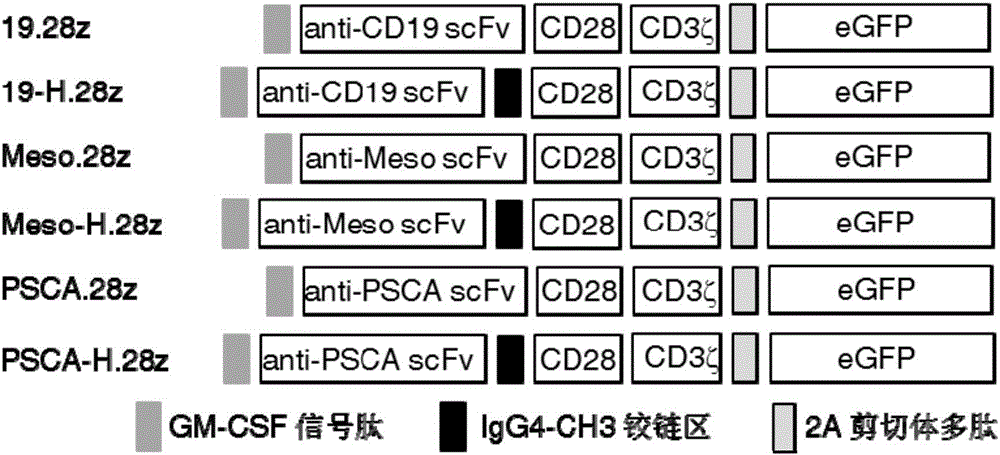
[0082] (1) Synthesize 19-H.28z, Meso-H.28z, PSCA-H.28z containing IgG4-CH3 hinge region and 19.28z, Meso.28z, PSCA.28z CAR molecular sequence without hinge region through whole gene synthesis , the C-terminus of the synthetic gene contains a restriction endonuclease PmeI restriction site, and the N-terminus contains a restriction endonuclease SpeI restriction site. The full-length gene map of the CAR molecule is as follows: figure 1 shown;
[0083] The nucleic acid sequence of the IgG4-CH3 hinge region is shown in SEQ ID NO.1:
[0084] Ggcggaggtagctctggcggtggatccggcgggcagccccgagaaccacaggtgtacaccctgcccccatcccgggatgagctgaccaagaaccaggtcagcctgacctgcctggtcaaaggcttctatcccagcgacatcgccgtggagtgggagagcaatgggcagccggagaacaactacaagaccacgcctcccgtgctggactccgacggctccttcttcctctacagcaagctcaccgtggacaagagcaggtggcagcaggggaacgtcttctcatgctccgtgatgcatgaggctctgcacaaccactacacgcagaagagcctctccctgtctccgggtaaa.
[0085] The nucleic acid sequen...
Embodiment 2
[0099] Example 2: Lentiviral packaging
[0100] When the cells cover 80-90% of the bottom of the 100mm culture dish, perform lentiviral packaging:
[0101] (1) 2 hours before virus packaging, the medium was replaced with DMEM containing 1% fetal bovine serum, 5mL / 100mm per culture dish;
[0102] (2) The packaging plasmids to be added to each 100mm culture dish of 293T cells are as follows:
[0103] Reagent dose pWPXLd-CAR-eGFP plasmid 4.5μg pMD2.G helper plasmid 1.5μg psPAX2 6μg
[0104] Add the plasmid to 500 μL opti-MEM medium and mix well;
[0105] Among them, pWPXLd-CAR-eGFP plasmids include: pwpxld-19.28z-eGFP, pwpxld-19-H.28z-eGFP, pwpxld-Meso.28z-eGFP, pwpxld-Meso-H.28z-eGFP, pwpxld-PSCA.28z -eGFP, pwpxld-PSCA-H.28z-eGFP;
[0106] (3) Add 36 μg PEI to another 500 μL opti-MEM medium, mix well, and let stand at room temperature for 5 minutes;
[0107] (4) Mix the plasmid with PEI, mix well by pipetting, and let stand at room tempe...
Embodiment 3
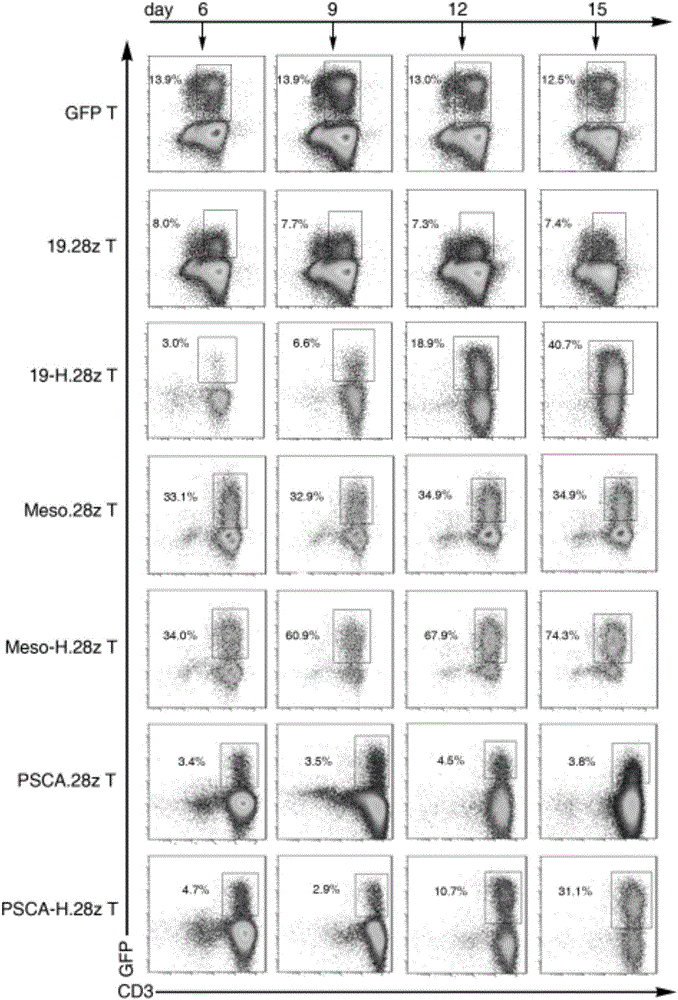
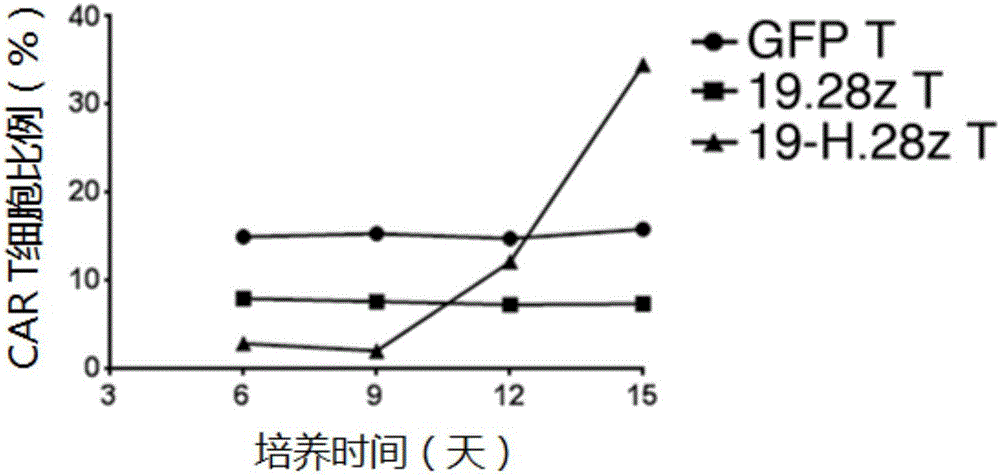
[0112] Example 3: Construction of CAR T cells
[0113] (1) Separation and purification of human T cells: Peripheral blood mononuclear cells (PBMC) were separated from whole blood by Ficoll (GE Company) density gradient centrifugation; ) to sort out T cells; T cells were activated for 72 hours using a T cell activation kit (Miltenyi);
[0114] (2) Lentiviral transfection of T cells: 72h after T cell activation, demagnetize the beads, resuspend the cells in 1640 medium containing 10% fetal bovine serum, 300IU / mLIL2, add the virus supernatant prepared in Example 2, every 10 6 Add 1mL virus supernatant to cells, polybrene 8μg / mL, at 37°C, 5% CO 2 After culturing in the incubator for 12 hours, resuspend with fresh medium containing 300IU / mL IL-2, every 10 6 Add 1 mL of culture medium to each cell.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com