Controlled-release nano medicine available for targeted application to neurodegenerative diseases
A neurodegenerative and disease-related technology, applied in the field of medicine, can solve the problems of drug limitations and inability to meet the needs of neurodegenerative diseases with high efficiency, sustained release, and targeted treatment, so as to reduce damage and burden, have no immunogenicity, The effect of high biosafety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] Embodiment 1: Preparation of nanoparticles loaded with acetylcholine (Ach)
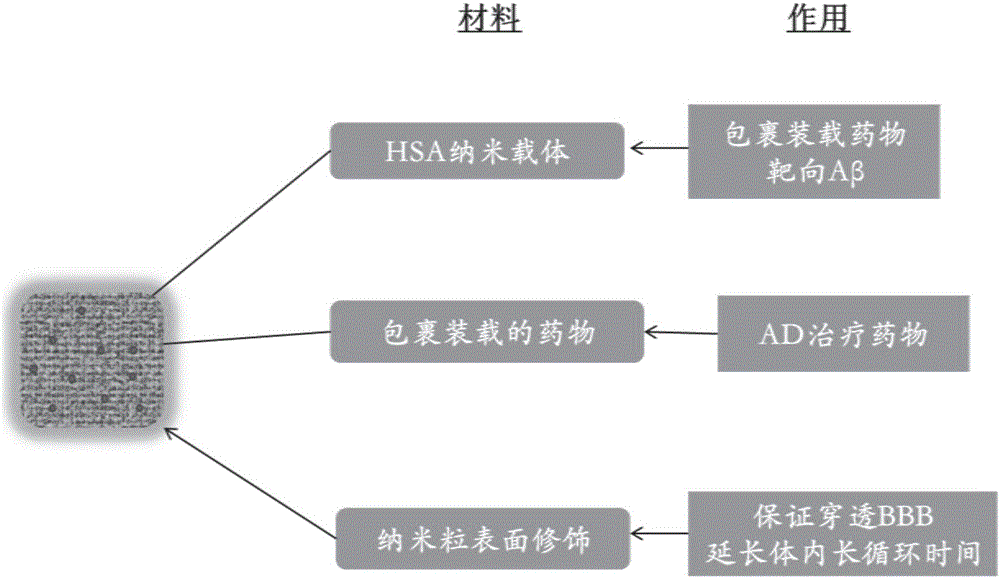
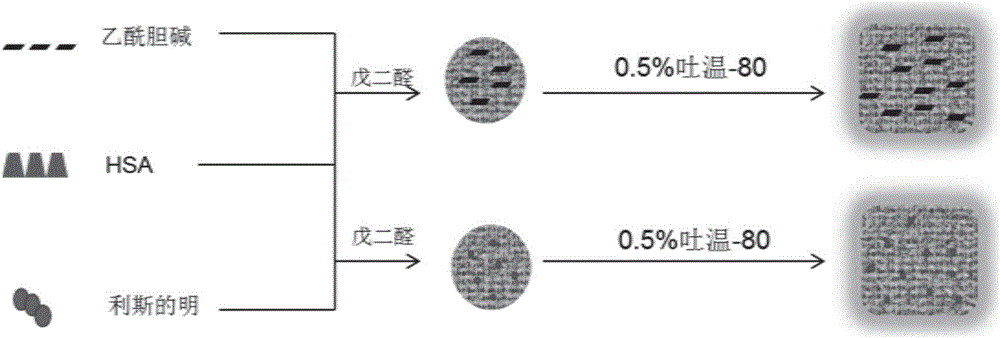
[0120] In this embodiment, human serum albumin (HSA) is used as the nano-carrier material to wrap and load acetylcholine. The active ingredient used is acetylcholine chloride, the good solvent selected is 10mmol / L sodium chloride solution, the poor solvent selected is ethanol, the cross-linking agent selected is 10% glutaraldehyde, and the targeted molecule selected is Wen-80 uses human serum albumin (HSA) nanoparticles as the carrier material. The preparation process is as follows.
[0121] Step (1): Prepare HSA drug-loaded nanoparticles by anti-solvent method: Dissolve 20mg HSA and 10mg ACh in 1mL sodium chloride solution (10mmol / L), filter with a 0.22μm water filter membrane, and at 600rpm, Add this solution dropwise to 10 mL of ethanol at a rate of 50 μL / min; after the dropwise addition, increase the stirring speed to 800 rpm, add 20 μL of 10% glutaraldehyde, and react in the dark for 24 ...
Embodiment 2
[0133] Embodiment 2: Preparation of nanoparticles loaded with rivastigmine (RT)
[0134] In this example, human serum albumin (HSA) nanoparticles were used as drug carriers to prepare nanoparticles loaded with rivastigmine (RT). The active ingredient used is rivastigmine bitartrate, the good solvent selected is the sodium chloride solution of 10mmol / L, the poor solvent selected is dehydrated alcohol (purity is greater than 99.7%), and the cross-linking agent selected is 10% Glutaraldehyde, the selected targeting molecule is Tween-80. The preparation process is as follows.
[0135] (1) Prepare HSA drug-loaded nanoparticles by anti-solvent method: dissolve 20mg HSA and 10mg RT in 1ml sodium chloride solution (10mmol / L), adjust the pH of the solution to 8.3 with 0.1mol / L sodium hydroxide solution, After filtering with a 0.22 μm water-based filter membrane, add the solution dropwise to 10 mL of absolute ethanol at a rate of 50 μL / min at a speed of 600 rpm; after the addition, in...
Embodiment 3
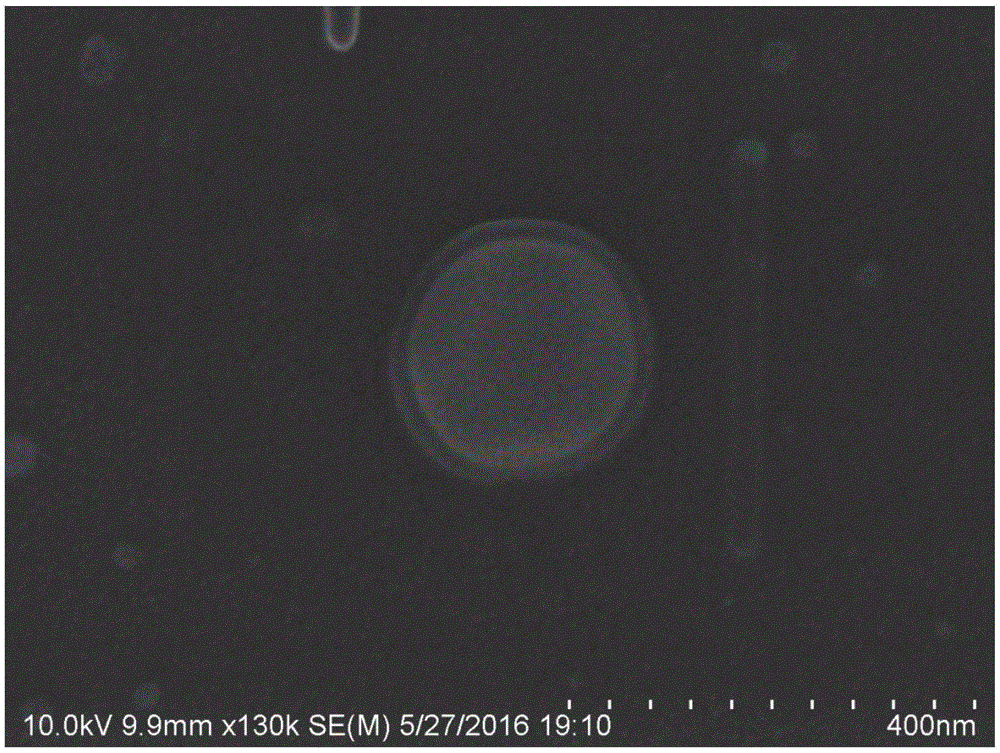
[0138] Example 3: Structural Characterization of Nanoparticles
[0139] (1) Ultraviolet absorption spectrum analysis
[0140] Respectively ACh aqueous solution, HSA aqueous solution, blank HSA nanoparticles that are not loaded with active ingredients and unmodified Tween-80 (the preparation process refers to the step (1) of Example 1), and the unmodified HSA nanoparticles prepared by the step (1) of Example 1. The HSA-ACh nanoparticles modified with Tween-80 were analyzed by ultraviolet absorption spectrum, and the ultraviolet absorption curve of the sample was as follows: Figure 6 shown. HSA solution has obvious characteristic absorption peak near 280nm (curve 1), but Ach solution has no absorption near 280nm (curve 2). After HSA forms blank nanoparticles, the absorption peak near 280nm is still clearly visible (curve 3); while the HSA nanoparticles loaded with Ach, the absorption peak near 280nm is significantly reduced (curve 4). Therefore, when HSA nanoparticles are do...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com