Preparation method of catalyst for preparing propylene by propane dehydrogenation and catalyst prepared by method
A propane dehydrogenation and catalyst technology, which is applied in chemical instruments and methods, physical/chemical process catalysts, hydrocarbons, etc., can solve the problems of complex preparation methods, weak interactions, catalyst deactivation, etc., to achieve stability and selection. The effect of improving the property, improving the interaction, and improving the dehydrogenation activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
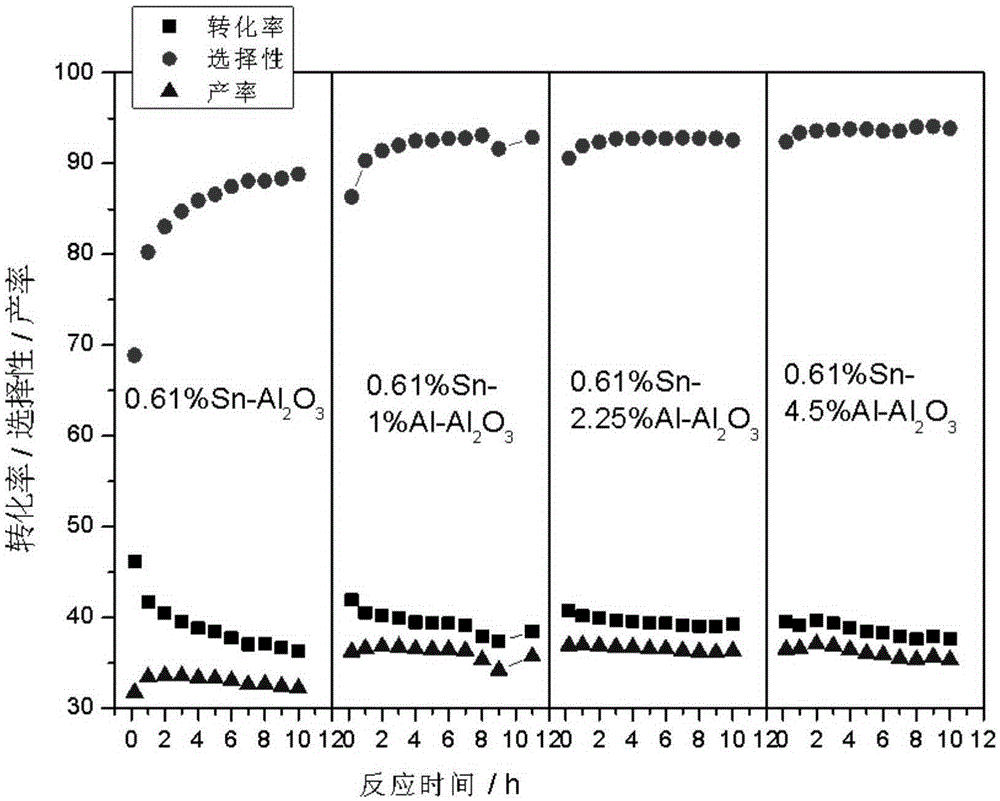
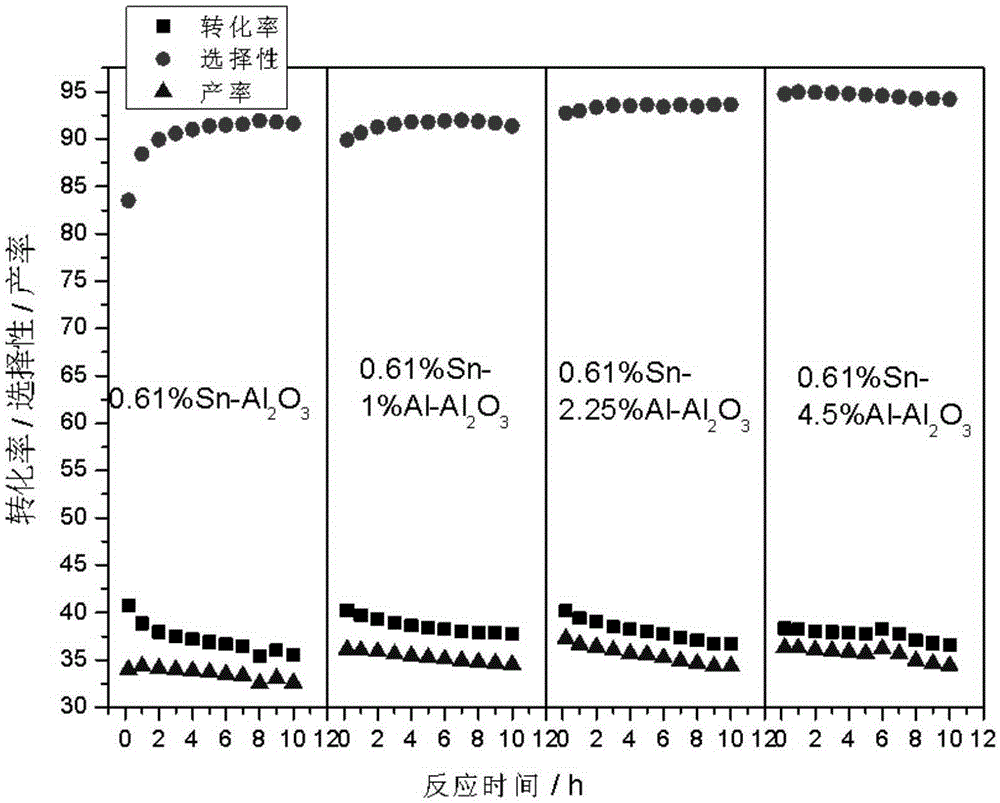
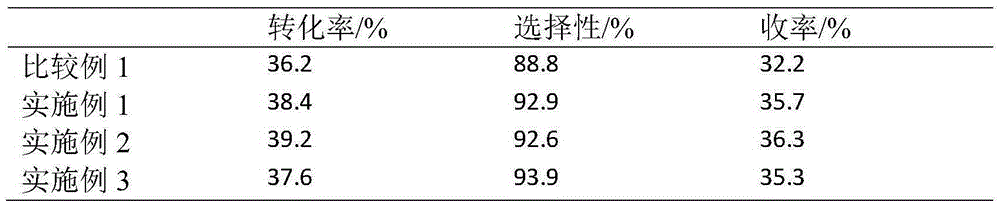
[0032] Preparation of Sn-Al / Al by Impregnation Method 2o 3 carrier. Take 2ml Al(NO 3 ) 3 (0.05gAl / ml) solution and 2.44ml (0.025gSn / ml) SnCl 4 Put the dilute hydrochloric acid solution into a 200ml beaker, add 20ml deionized water, and mix well. Weigh 10 g of alumina A pellets and add to the above solution, let it stand for adsorption for 4 hours. Dry at 60°C for 4 hours, dry at 120°C for 4 hours, and calcinate at 550°C for 12 hours to obtain a catalyst carrier with dual promoters.
[0033] Take 10g of the above-mentioned carrier and put it into a 250ml suction filter bottle, and vacuumize for 0.5h. Add 1.35ml (0.037gPt / ml) of chloroplatinic acid solution and 2.4ml of 10% dilute hydrochloric acid. Dry at 60°C for 4 hours, dry at 120°C for 4 hours, and bake at 550°C for 4 hours.
[0034] Then the prepared catalyst was steam treated at 550°C for 4h; then impregnated with 5.6ml KNO 3 (0.025gK / ml) solution was introduced with alkali metal additives, the sample was dried a...
Embodiment 2
[0038] Preparation of Sn-Al / Al by Impregnation Method 2 o 3 carrier. Take 4.5ml Al(NO 3 ) 3 (0.05gAl / ml) solution and 2.44ml (0.025gSn / ml) SnCl 4 Put the dilute hydrochloric acid solution into a 200ml beaker, add 20ml deionized water, and mix well. Weigh 10 g of alumina A pellets and add to the above solution, let it stand for adsorption for 4 hours. Dry at 60°C for 4 hours, dry at 120°C for 4 hours, and calcinate at 550°C for 12 hours to obtain a catalyst carrier with dual promoters.
[0039] Take 10g of the above-mentioned carrier and put it into a 250ml suction filter bottle, and vacuumize for 0.5h. Add 1.35ml (0.037gPt / ml) of chloroplatinic acid solution and 2.4ml of 10% dilute hydrochloric acid. Dry at 60°C for 4 hours, dry at 120°C for 4 hours, and bake at 550°C for 4 hours.
[0040] Then the prepared catalyst was steam treated at 550°C for 4h; then impregnated with 5.6ml KNO 3 (0.025gK / ml) solution was introduced with alkali metal additives, the sample was drie...
Embodiment 3
[0044] Preparation of Sn-Al / Al by Impregnation Method 2 o 3 carrier. Take 9ml Al(NO 3 ) 3 (0.05gAl / ml) solution and 2.44ml (0.025gSn / ml) SnCl 4 Put the dilute hydrochloric acid solution into a 200ml beaker, add 20ml deionized water, and mix well. Weigh 10 g of alumina A pellets and add to the above solution, let it stand for adsorption for 4 hours. Dry at 60°C for 4 hours, dry at 120°C for 4 hours, and calcinate at 550°C for 12 hours to obtain a catalyst carrier with dual promoters.
[0045] Take 10g of the above-mentioned carrier and put it into a 250ml suction filter bottle, and vacuumize for 0.5h. Add 1.35ml (0.037gPt / ml) of chloroplatinic acid solution and 2.4ml of 10% dilute hydrochloric acid. Dry at 60°C for 4 hours, dry at 120°C for 4 hours, and bake at 550°C for 4 hours.
[0046] Then the prepared catalyst was steam treated at 550°C for 4h; then impregnated with 5.6ml KNO 3 (0.025gK / ml) solution was introduced with alkali metal additives, the sample was dried ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com