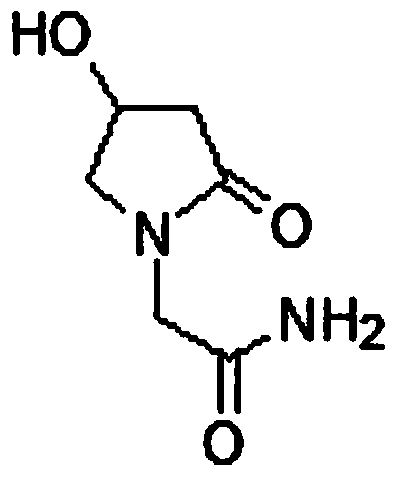
A method for preparing oxiracetam oral film by hot-melt extrusion
An oral film and hot-melt technology, applied in the field of oxiracetam, can solve the problems of difficult control of disintegration time and tensile strength, restrictions on the development and application of oral film, and complex liposome preparation process, etc., to achieve Increased bioavailability, avoid elimination effects, uniform and complete appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
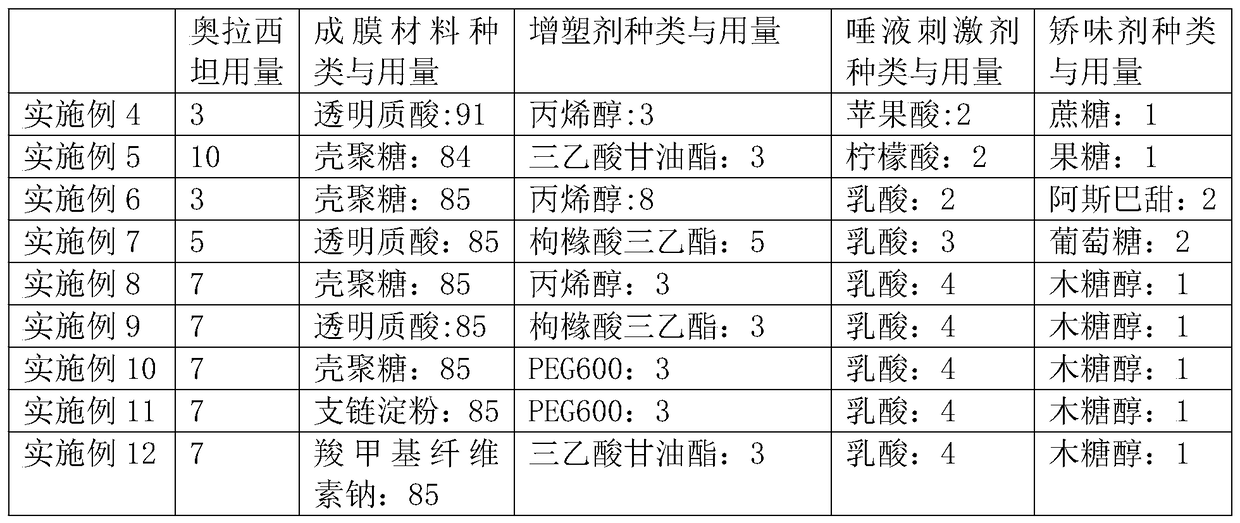
[0038] Mix 6g oxiracetam, 87g chitosan, 4g propylene alcohol, 2g malic acid, and 1g fructose, fully grind and mix evenly, then send it to the hot melt zone through the feed zone of the hot melt laminator, and place it at 80- Hot melt at 85°C, the molten mixture is continuously output through the metering area, poured into the mold, and forms a film after cooling.
Embodiment 2
[0040] Mix 7g oxiracetam, 85g chitosan, 5g propylene alcohol, 2g citric acid, and 1g glucose, fully grind and mix evenly, and then send it to the hot-melt zone through the feed zone of the hot-melt film laminating machine. Hot melt at 90°C, the molten mixture is continuously output through the metering area, poured into the mold, and forms a film after cooling.
[0041] The hot-melt lamination process can be carried out with reference to the following documents: Repka MA, Battu SK, Upadhye SB, etal.Pharmaceutical applications of hot-melt extrusion: part II[J].Drug Dev IndPharm, 2007,33(10):1043-1057 .
Embodiment 3
[0043] Mix 8g of oxiracetam, 86g of hyaluronic acid, 3g of triethyl citrate, 2g of saliva stimulant, and 1g of xylitol. After fully grinding and mixing evenly, send it to the The hot melting zone is hot-melted at 85-90°C, and the molten mixture is continuously output through the metering zone, poured into the mold, and forms a film after cooling.
[0044] With reference to embodiment 1-3, prepare following embodiment: (consumption is weight g in the following table)
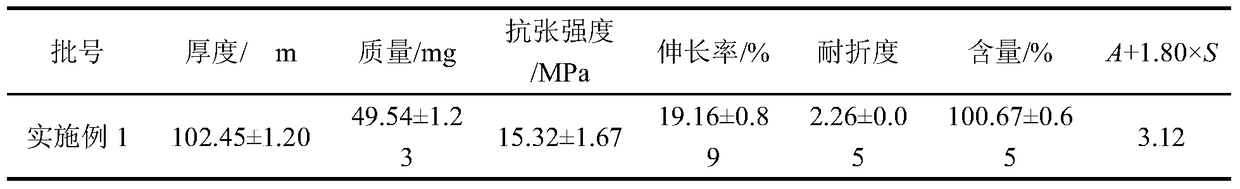
[0045]
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com