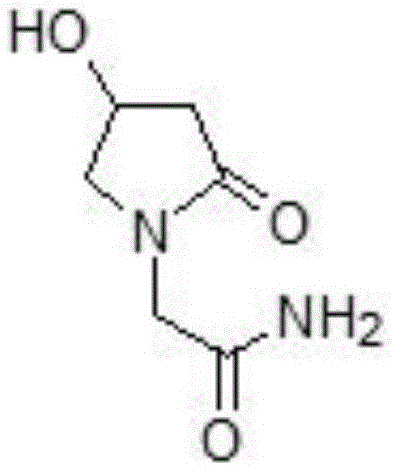
Oxiracetam oral membrane preparation and preparation method thereof
An oral film and filler technology, which is applied in the field of oxiracetam oral film and its preparation, can solve the problem that disintegration time and tensile strength are not easy to control, restrict the development and application of oral film, and prepare liposomes. Complex process and other problems, to achieve the effect of improving bioavailability, avoiding elimination effect, and uniform and complete appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] A preparation method of oxiracetam oral film, adopts following steps:
[0083] 1) Dissolve 40-65 parts of ethyl cellulose in absolute ethanol to form a homogeneous viscous liquid, then add 10-25 parts of glycerin and mix well to form material I;
[0084] 2) Mix 1-20 parts of oxiracetam and 5-30 parts of filler, and disperse with absolute ethanol to form material II;
[0085] 3) Mix the material II with the material I, and stir at a stirring rate of 500r / min to 800r / min for 60min to 120min to form oxiracetam bubble-free suspension viscous liquid;
[0086] 4) Cast the oxiracetam bubble-free suspension viscous liquid prepared in step 3) on a mold, dry at 55-90° C., cool at room temperature, form a film, and cut to obtain the oxiracetam oral film.
Embodiment 2
[0088] A preparation method of levoxiracetam oral film, adopts following steps:
[0089] 1) Dissolve 35-60 parts of ethyl cellulose in purified water to form a homogeneous viscous liquid, then add 15-30 parts of propylene glycol and mix to form material I;
[0090] 2) Mix 1-20 parts of levoxiracetam and 5-30 parts of filler, and disperse with purified water to form material II;
[0091] 3) Mix material II with material I, and stir for 70-130 min at a stirring rate of 500r / min to 700r / min to form levoxiracetam suspension viscous liquid without bubbles;
[0092] 4) Cast the levo-oxiracetam bubble-free suspension viscous liquid prepared in step 3) on a mold, dry at 45-85°C, cool at room temperature, form a film, and cut to obtain the levo-oxiracetam oral film agent.
Embodiment 3
[0094]
[0095] 1) Dissolving ethyl cellulose in absolute ethanol to form a homogeneous viscous liquid, then adding glycerin and mixing to form material I;
[0096] 2) Mix oxiracetam and microcrystalline cellulose, and disperse with absolute ethanol to form material II;
[0097] 3) Mix the material II with the material I, stir, the stirring speed is 500r / min~600r / min, the stirring time is 80min~90min, and form oxiracetam non-bubble suspension viscous liquid;
[0098] 4) Cast the oxiracetam bubble-free suspension viscous liquid obtained in step 3) on a mold, dry at 72-75° C., cool at room temperature, form a film, and cut to obtain the oxiracetam oral film.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com