Process for preparing high-purity minodronate
A technology of minodronic acid and high purity is applied in the field of preparing high-purity minodronic acid to achieve the effects of improving human health, simple operation and avoiding hip fractures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
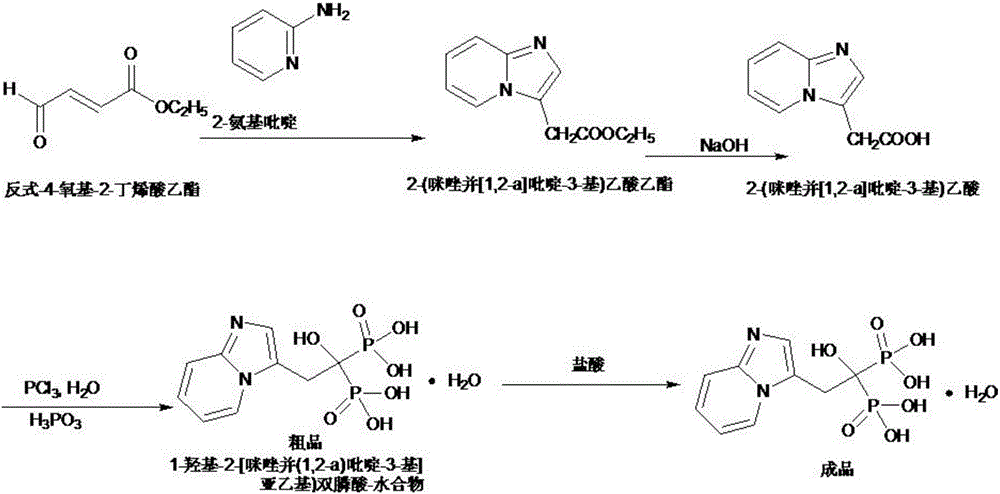
[0022] Such as figure 1 Shown, a kind of technique for preparing high-purity minodronic acid of the present invention comprises the following processing steps:
[0023] (1) Add 100g of 2-aminopyridine to 1000g of methanol, dropwise add 120g of trans-4-oxyl-2-butenoic acid ethyl ester to react, after about 30min, heat to 85±5℃ for reaction, After completion of the reaction, cool to room temperature to generate 2-(imidazo[1,2-a]-3-yl)ethyl acetate, add 1100g of 10% NaOH for hydrolysis, and concentrate to obtain 2-(imidazo[1,2-a ]-3-yl)acetic acid;
[0024] (2) Add 1200g toluene and 150g phosphorous acid to the solution containing 2-(imidazo[1,2-a]pyridin-3-yl)acetic acid, stir and heat to react, then add 420g phosphorus trichloride dropwise to react, cool , to obtain the crude product of minodronic acid, the heating temperature is 50-120°C.
[0025] (3) Add 5000 g of 1 mol / L hydrochloric acid to the solution containing the crude minophosphoric acid, heat to reflux until compl...
Embodiment 2
[0027] A kind of technique for preparing high-purity minodronic acid of the present invention comprises the following process steps:
[0028] (1) Add 92g of 2-aminopyridine to 1000g of absolute ethanol, add 110g of trans-4-oxyl-2-butenoic acid ethyl ester dropwise to react, drop for about 30min, and heat to 85±5°C After the reaction is complete, cool to room temperature to generate 2-(imidazo[1,2-a]-3-yl)ethyl acetate, add 1000g of 10% NaOH for hydrolysis, and concentrate to obtain 2-(imidazo[1,2 -a]-3-yl)acetic acid;
[0029] (2) Add 1000g of toluene and 130g of phosphorous acid to the solution containing 2-(imidazo[1,2-a]pyridin-3-yl)acetic acid, stir and heat for reaction, then add 380g of phosphorus trichloride dropwise for reaction, and cool , to obtain the crude product of minodronic acid, the heating temperature is 110°C to 120°C;
[0030] (3) Add 4600 g of 1 mol / L hydrochloric acid to the solution containing the crude minophosphoric acid, heat to reflux until complet...
Embodiment 3
[0032] A kind of technique for preparing high-purity minodronic acid of the present invention comprises the following process steps:
[0033] (1) Add 85g of 2-aminopyridine to 1000g of anhydrous n-butanol, add 100g of trans-4-oxyl-2-butenoic acid ethyl ester dropwise to react, drop for about 30min, and heat to 85± React at 5°C, after the reaction is complete, cool to room temperature to generate 2-(imidazo[1,2-a]-3-yl)ethyl acetate, add 1000g of 10% NaOH for hydrolysis, and concentrate to obtain 2-(imidazo[1,2-a] ,2-a]-3-yl)acetic acid;
[0034] (2) Add 900g of toluene and 110g of phosphorous acid to the solution containing 2-(imidazo[1,2-a]pyridin-3-yl)acetic acid, stir and heat for reaction, then add 350g of phosphorus trichloride dropwise for reaction, and cool , to obtain the crude product of minodronic acid, the heating temperature is 110°C to 120°C;
[0035] (3) Add 4500 g of 1 mol / L hydrochloric acid to the solution containing the crude minophosphoric acid, heat to re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com