High sensitivity analysis method of genotoxic impurities in nintedanib ethanesulfonate
A technology for nintedanib ethanesulfonate and genotoxicity, which is applied in the field of analysis of genotoxic impurities in nintedanib ethanesulfonate, can solve unseen and other problems, and achieves simple operation, low cost, efficient separation and detection Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
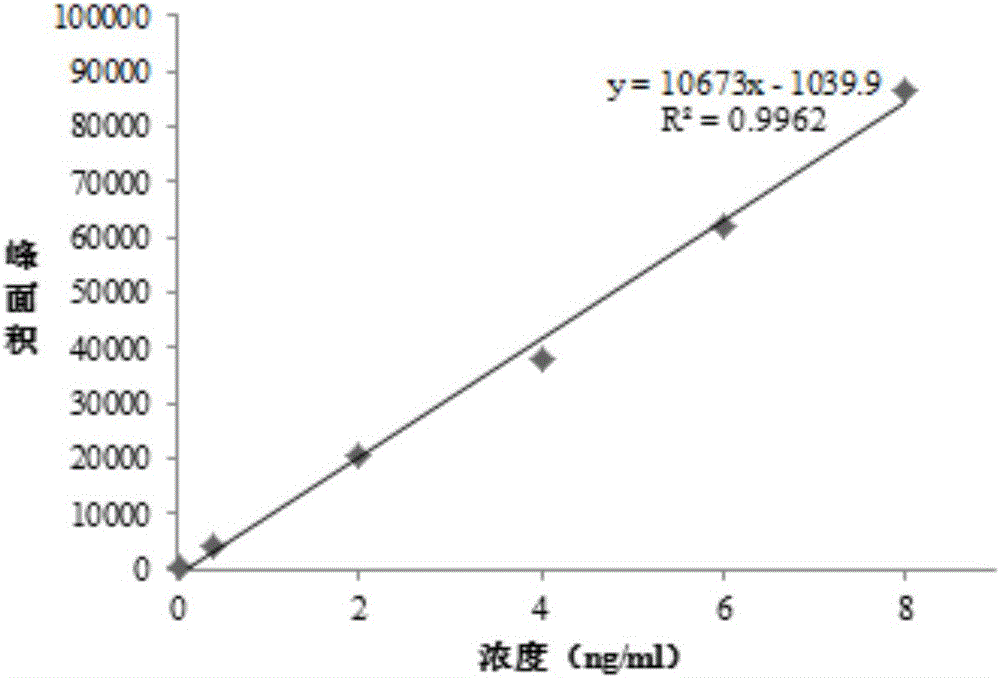
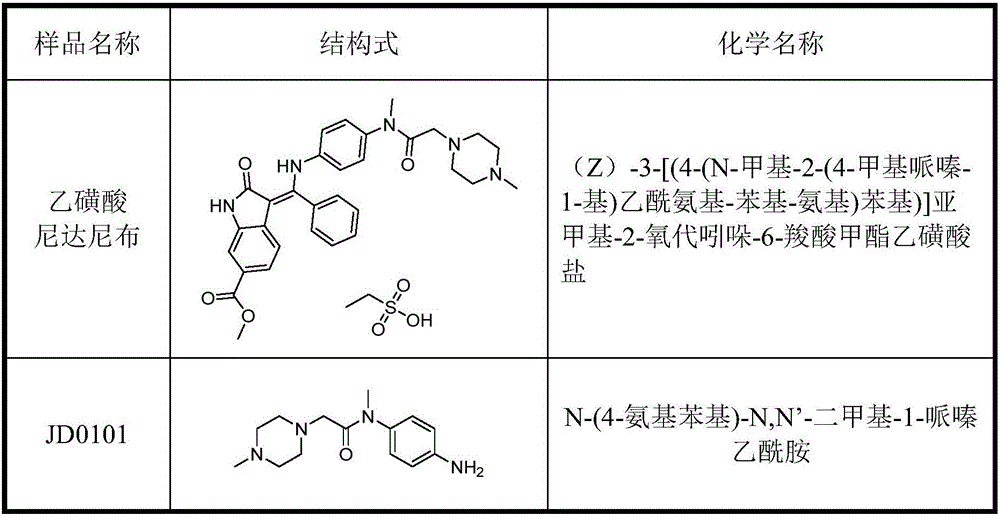
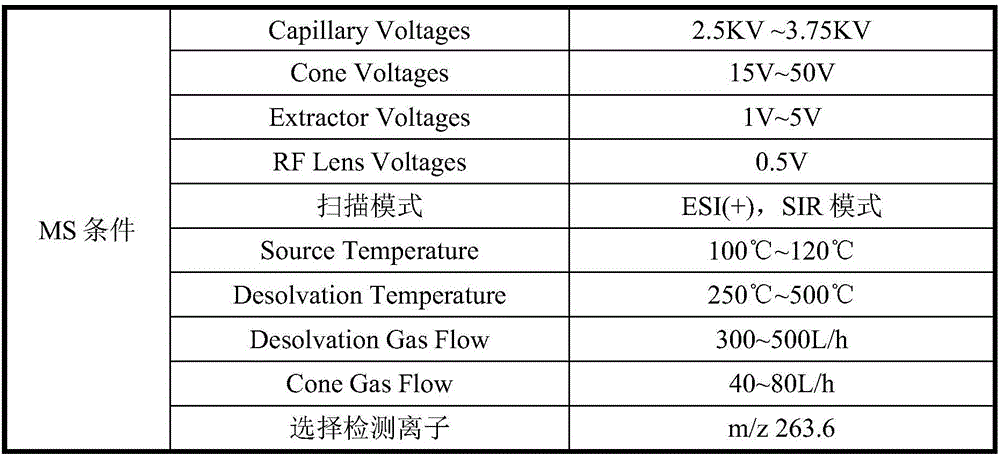
[0035] Example 1: Analysis of nintedanib ethanesulfonate and genotoxic impurity JD0101 by high performance liquid chromatography-mass spectrometry tandem method.
[0036] 1. Preparation of standard stock solution
[0037] Accurately weigh about 10mg of impurity JD0101, put it in a 100mL measuring bottle, dissolve it with methanol and dilute to the mark to make a solution containing about 0.1mg of JD0101 per 1mL as JD0101 standard stock solution Ⅰ. Precisely pipette 0.1mL of JD0101 standard stock solution Ⅰ, put it in a 100mL volumetric flask, dilute to the mark with methanol, and make standard stock solution Ⅱ containing 0.1μg of JD0101 per 1mL. Then dissolve and gradually dilute to the desired concentration with methanol-water (70:30V / V).
[0038] Two, the test solution
[0039] Accurately weigh about 20 mg of the test product, put it in a 50 mL measuring bottle, dissolve and dilute it with methanol-water (70:30 V / V) to make a solution containing about 0.4 mg per 1 mL as th...
Embodiment 2
[0069] Example 2: High-performance liquid chromatography analysis of nintedanib ethanesulfonate and genotoxic impurity JD0101
[0070] 1. Preparation of standard stock solution
[0071] Accurately weigh about 10mg of impurity JD0101, put it in a 100mL measuring bottle, dissolve it with methanol and dilute to the mark to make a solution containing about 0.1mg of JD0101 per 1mL as JD0101 standard stock solution Ⅰ. Precisely pipette 1mL of JD0101 stock solution Ⅰ, put it in a 100mL measuring bottle, dilute to the mark with methanol, and make standard stock solution Ⅱ containing 1μg of JD0101 per 1mL. Afterwards, gradually dilute to the desired concentration with methanol-water (70:30V / V).
[0072] 2. Instrument and liquid chromatography conditions
[0073] Waters Empower3 chromatographic workstation, automatic sampler, column thermostat. The chromatographic column is ShimadzuInertSustain C18 (4.6*150mm, 5μm), the flow rate is 0.4mL / min, the detection wavelength is 242nm, the c...
Embodiment 3
[0084] 1, the preparation of standard stock solution is identical with embodiment 1;
[0085] 2, need testing solution is identical with embodiment 1;
[0086] 3. Instrument and chromatographic conditions: the temperature of the sample chamber is 5°C, and the rest are the same as in Example 1;
[0087] 4. The methodological verification is the same as in Example 1, and the results show that the average recovery rate of impurity JD0101 (according to the Pharmacopoeia regulation 80-115%) is 99.9%, and the RSD is 3.6%, showing that the accuracy of the method for detecting impurity JD0101 is very good.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com