Quality control method of powerful dysentery relieving soluble powder preparation
A quality control method and soluble technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as inability to fully and effectively control the quality of Laotie Zhili soluble powder preparations, affecting product production and sales, quality assurance, and unquestionable curative effect , to achieve the effect of ensuring internal quality and curative effect, ensuring controllability and optimizing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] ①Preparation of the test solution: Take 5g of Laotiezhidysentery soluble powder sample, add 25ml of methanol, ultrasonicate for 30min, filter, evaporate the filtrate to dryness, add 3ml of methanol to dissolve, and use it as the test solution;
[0053] ②Preparation of reference substance solution: take berberine hydrochloride and palmatine hydrochloride reference substances, add methanol to make a mixed solution containing 0.2mg per 1ml, as the reference substance solution;
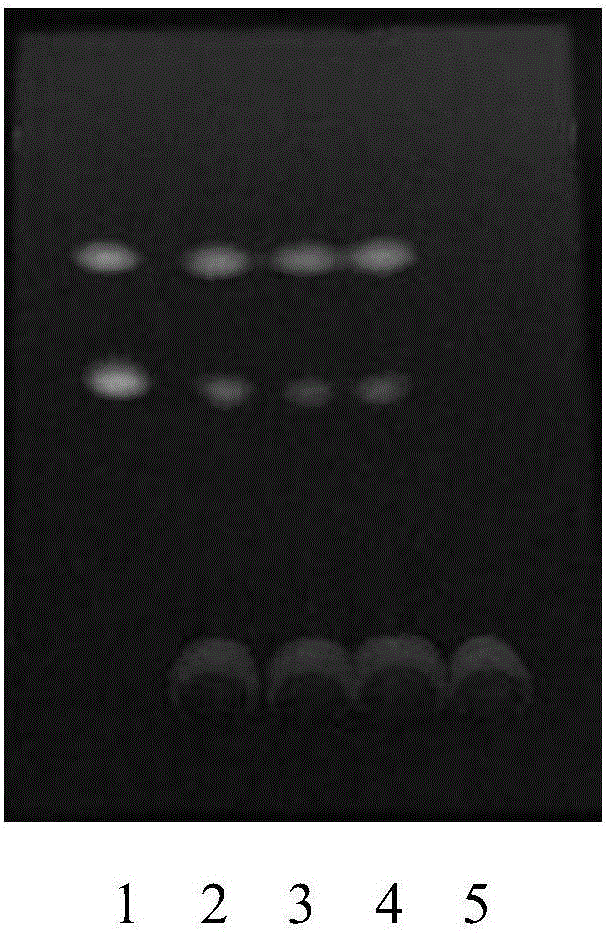
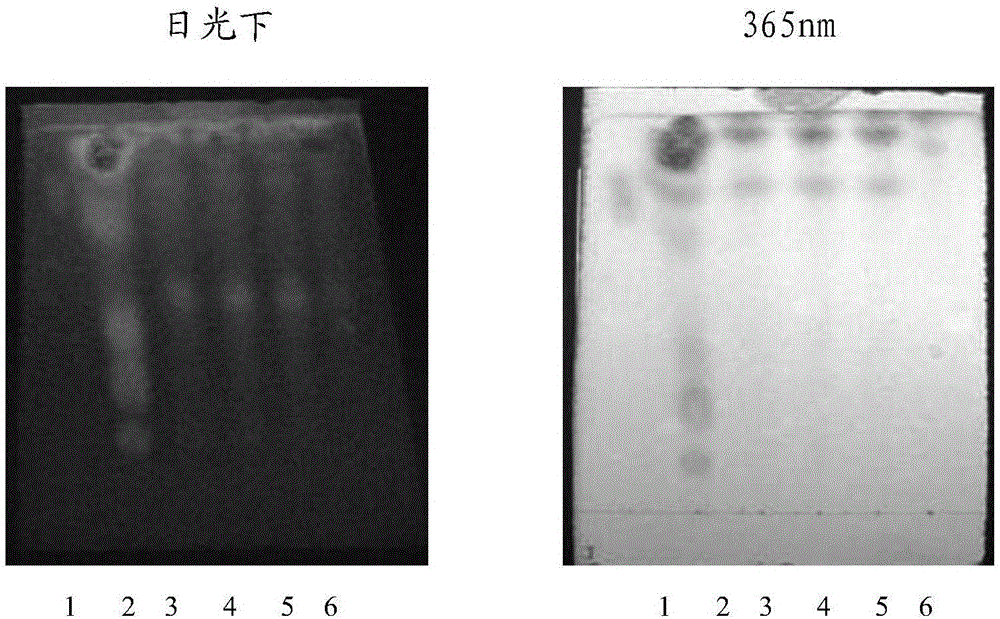
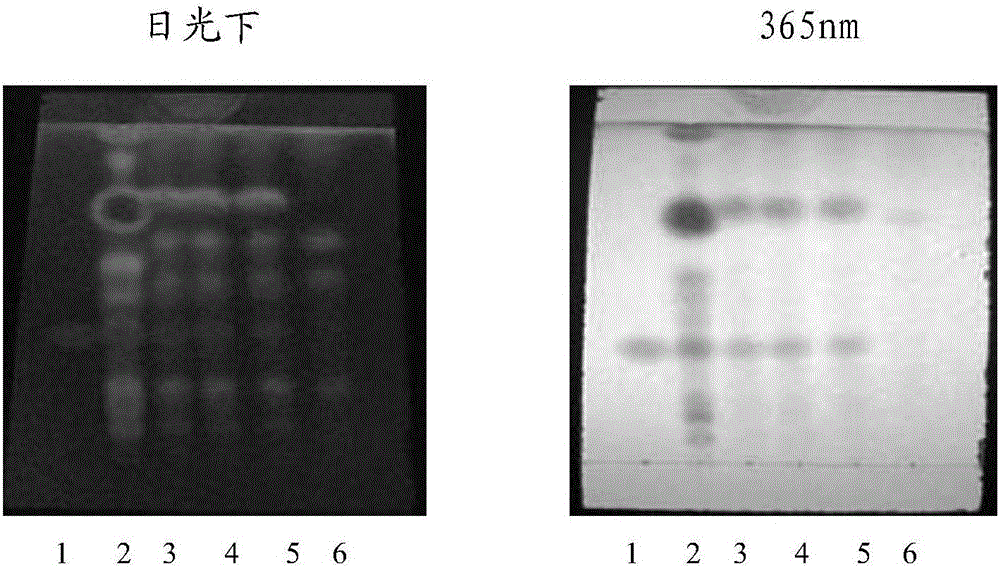
[0054] ③ Identification: Take 5 μl of the two solutions of the test solution and the reference solution, respectively, and place them on the same silica gel G thin-layer plate, and use benzene-ethyl acetate-methanol-isopropanol-concentrated ammonia test solution according to the ratio of 6:3 : 1.5:1.5:0.5 mass fraction ratio is mixed as developing agent, puts in the chromatographic cylinder saturated with ammonia vapor, develops, takes out, dries, puts inspection under 365nm ultraviolet light lamp, ...
Embodiment 1
[0069] Example 1 Test
[0070] A kind of quality control method of old iron antidiarrheal soluble powder preparation, the steps of its method are:
[0071] 1. Identification project test:
[0072] 1 TLC identification test of ten major contributions:
[0073] Laotie Zhili soluble powder quality standard identification for TLC identification of the top ten contributions, the test is as follows:
[0074] 1.1 Experimental materials:
[0075] Berberine hydrochloride reference substance: China Food and Drug Control Institute provides (110713-201212 for content determination);
[0076] Palmatine hydrochloride reference substance: provided by China National Institutes for Food and Drug Control (110732-201510 for content determination);
[0077] Silicone G thin-layer board: Qingdao Shenghai Fine Silicone Chemical Co., Ltd., 5×10cm.
[0078] 1.2 Preparation of the test solution:
[0079] Take 5g of Laotiezhidiarrhea soluble powder sample, add 25ml of methanol, ultrasonicate for 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com