Norbornene type compound, and preparation method and application thereof
A norbornene and compound technology, applied in the field of norbornene derivatives, can solve the problems of limited wide application, swelling, poor dimensional stability, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
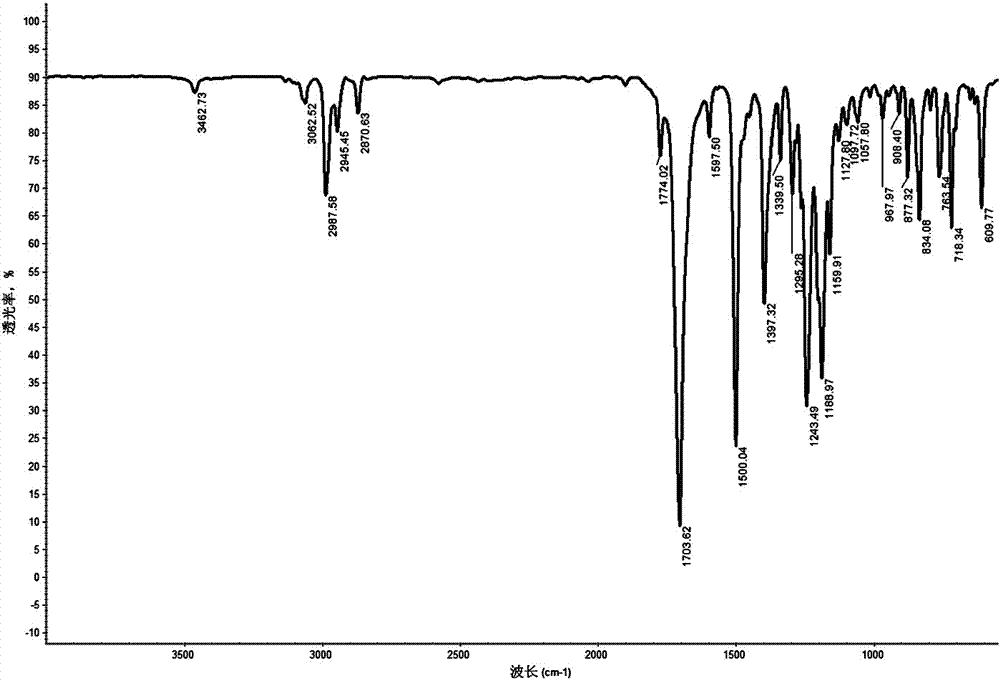
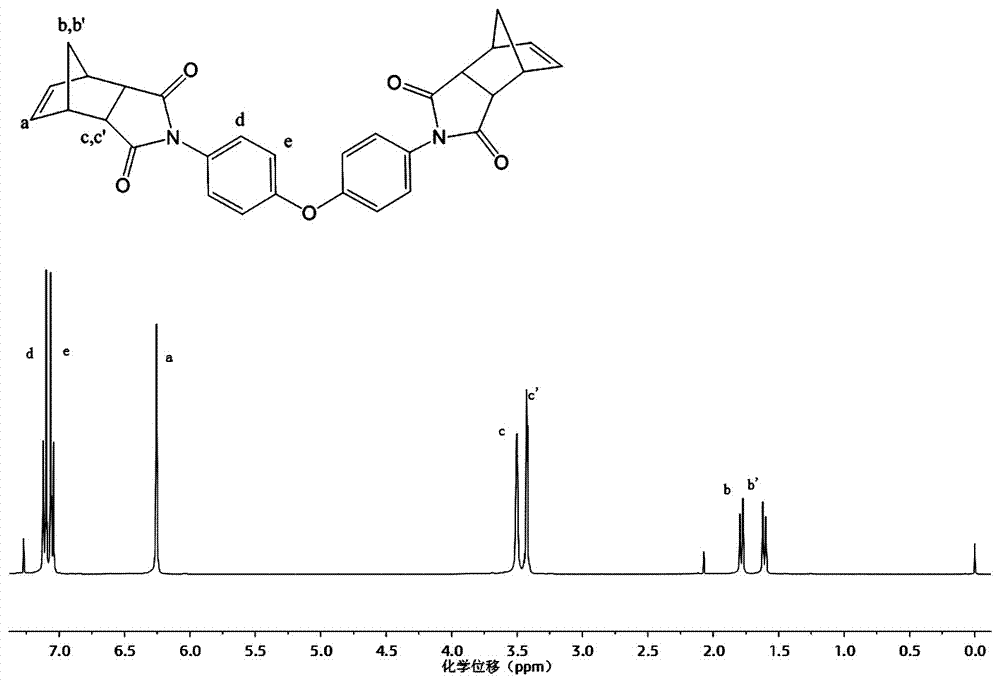
[0038] Add 200g of norbornene diacid anhydride into 500ml of glacial acetic acid, stir and dissolve evenly, raise the temperature to 120°C, slowly add 100g of 4,4'-diaminodiphenyl ether while stirring, condense and heat under reflux for 12h. Deionized water was added to the reaction solution to precipitate a white precipitate, which was filtered by suction and dried in vacuo to obtain 280 g of a crosslinking agent intermediate 4,4'-bis(norbornene-5,6-diamidyl)-p-phenylene ether white powder, Yield 95%.
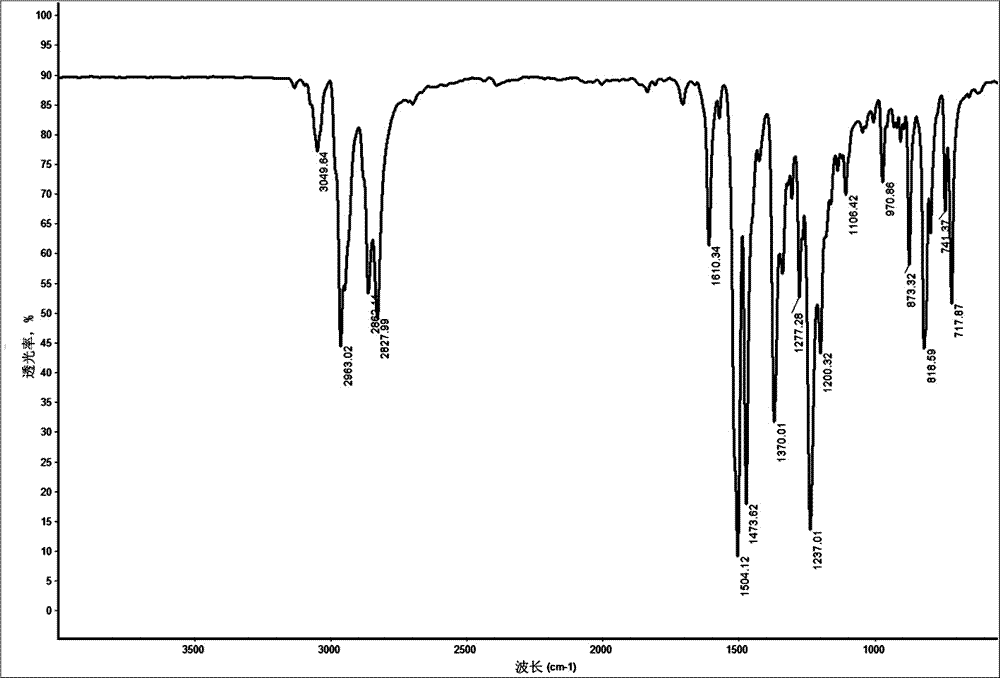
[0039] Add 200g of lithium aluminum tetrahydride to 200ml of ether, stir and mix well. Take 200g of 4,4'-bis(norbornene-5,6-diamido)-p-phenylene ether and dissolve it in 200ml of dichloromethane, and slowly add it dropwise to the ether solution of lithium aluminum hydride mentioned above at 0°C, at room temperature Under reaction 24h. The reaction solution was suction filtered to obtain a colorless solution, added anhydrous magnesium sulfate to dry, suction filtered, and rot...
Embodiment 2
[0041] Add 250g of norbornene diacid anhydride to 500ml of glacial acetic acid, stir and dissolve evenly, raise the temperature to 120°C, slowly add 100g of 4,4'-diaminodiphenyl ether while stirring, condense and heat under reflux for 12h. Deionized water was added to the reaction solution to precipitate a white precipitate, which was filtered by suction and dried in vacuo to obtain 350 g of a crosslinking agent intermediate 4,4'-bis(norbornene-5,6-diamidyl)-p-phenylene ether white powder, Yield 93%.
[0042]Add 200g of lithium aluminum tetrahydride to 200ml of ether, stir and mix well. Take 200g of 4,4'-bis(norbornene-5,6-diamido)-p-phenylene ether and dissolve it in 200ml of dichloromethane, and slowly add it dropwise to the ether solution of lithium aluminum hydride mentioned above at 0°C, at room temperature Under reaction 24h. The reaction solution was filtered with suction to obtain a colorless solution, dried by adding anhydrous magnesium sulfate, filtered with suctio...
Embodiment 3
[0044] Add 300g of norbornene diacid anhydride to 500ml of glacial acetic acid, stir and dissolve evenly, raise the temperature to 120°C, slowly add 100g of 4,4'-diaminodiphenyl ether while stirring, condense and heat under reflux for 12h. Deionized water was added to the reaction solution to precipitate a white precipitate, which was filtered by suction and dried in vacuo to obtain 400 g of a crosslinking agent intermediate 4,4'-bis(norbornene-5,6-diamidyl)-p-phenylene ether white powder, Yield 88%.
[0045] Add 200g of lithium aluminum tetrahydride to 200ml of ether, stir and mix well. Take 250g of 4,4'-bis(norbornene-5,6-diamido)-p-phenylene ether and dissolve it in 200ml of dichloromethane, and slowly add it dropwise to the ether solution of lithium aluminum hydride mentioned above at 0°C, at room temperature Under reaction 24h. The reaction solution was filtered with suction to obtain a colorless solution, dried by adding anhydrous magnesium sulfate, filtered with sucti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com