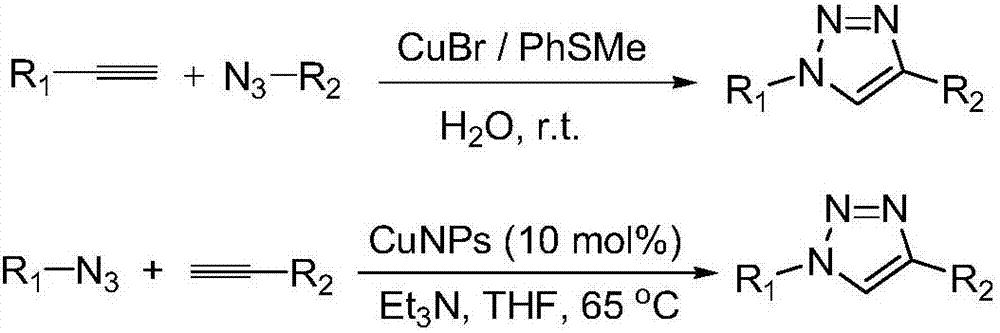
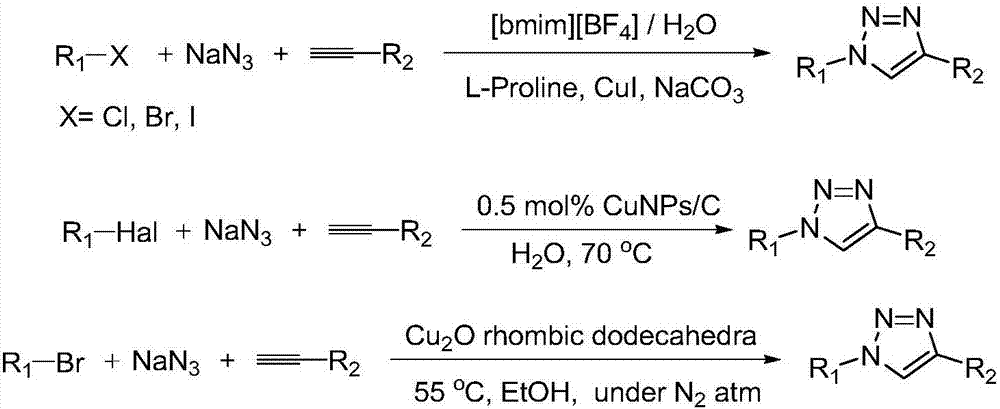
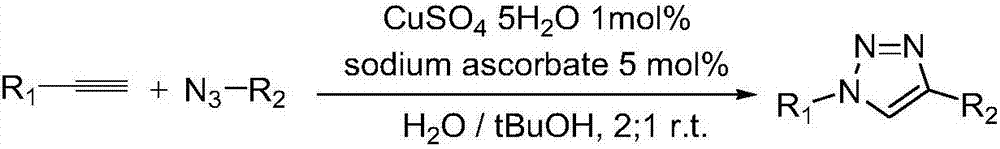Method for preparing 1, 4-substituted-1, 2, 3-triazole from (Z)-beta-alkenyl bromide multicomponents and use thereof
A technology for alkenyl bromide and triazole, which is applied in the field of preparation of 1,2,3-triazole and achieves the effects of short route, high yield and fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Add 1.0mmol of phenylpropane (Z)-β-alkenyl bromide, 1.2mol of sodium azide, 1.5mmol of m-trifluorobenzyl bromide, 3.0mmol of DBU, and 0.2mmol of cuprous iodide to polyethylene glycol In alcohol 4003.0mL, stirred at 100°C for 30 minutes, after the reaction, the product was subjected to column chromatography V 石油醚 :V 乙酸乙酯 = 3:1 treatment gives 1-(3-trifluoromethylbenzyl)-4-phenyl-1,2,3-triazole. The product is a white solid, yield: 98%.
Embodiment 2
[0045] Example 2: Add 1.0 mmol of phenylpropane (Z)-β-alkenyl bromide, 1.2 mol of sodium azide, 1.5 mmol of m-trifluorobenzyl bromide, and 0.1 mmol of cuprous iodide to 400 3.0 mL of polyethylene glycol , stirred at 100°C for 2.0h, after the reaction, the product was subjected to column chromatography V 石油醚 :V 乙酸乙酯 = 3:1 treatment gives 1-(3-trifluoromethylbenzyl)-4-phenyl-1,2,3-triazole. The product is a white solid, yield: 89%.
Embodiment 3
[0046] Example 3: Add 1.0 mmol of phenylpropane (Z)-β-alkenyl bromide, 1.2 mol of sodium azide, 1.5 mmol of m-trifluorobenzyl bromide, and 0.1 mmol of cuprous iodide to 400 3.0 mL of polyethylene glycol , stirred at room temperature and reacted for 25h, after the reaction, the product was subjected to column chromatography V 石油醚 :V 乙酸乙酯 = 3:1 treatment gives 1-(3-trifluoromethylbenzyl)-4-phenyl-1,2,3-triazole. The product is a white solid, yield: 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com