Synthesis method of phenoxazine compounds or phenothiazine compounds
A synthesis method and compound technology, applied in the field of oxygen and nitrogen compound synthesis, can solve the problems of narrow amine source selection range, poor functional group tolerance, harsh reaction conditions, etc., and achieve expansion tolerance, good yield, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
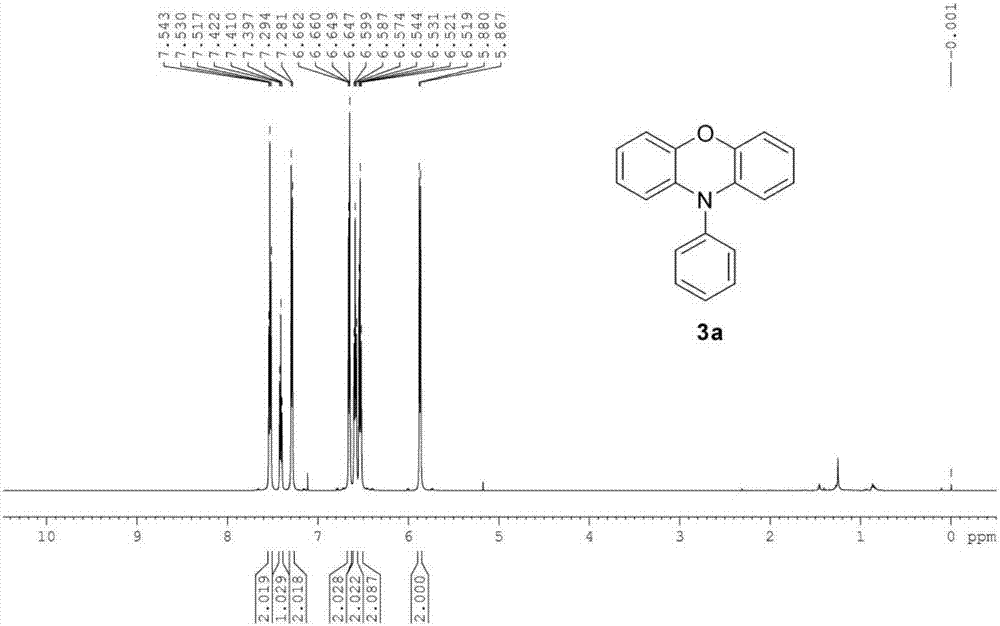
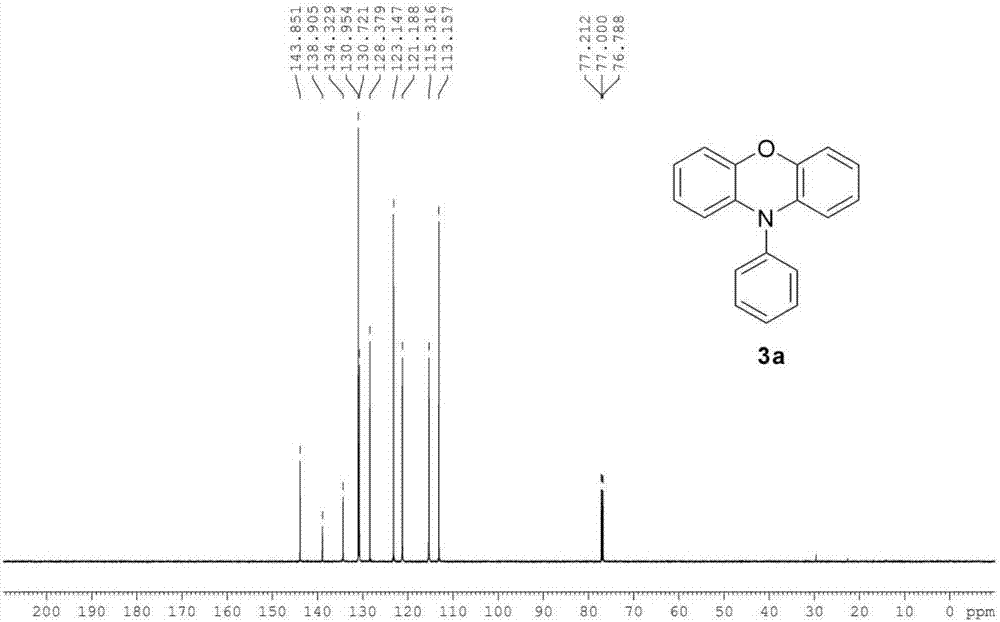
[0030] The preparation method of the phenoxazine compound A of following structural formula:
[0031]
[0032] To a dry 25 mL Schlenk reaction tube was added the dihalide compound 2,2'-dibromodiphenyl ether (0.2 mmol), followed by aniline (0.22 mmol), Pd(OAc) 2 (0.01mmol, 5mol%), DPEphos (0.02mmol, 10mol%), sodium tert-butoxide (0.6mmol), under the protection of nitrogen, add toluene (2mL), and react at 120°C for 15h. After the reaction, the target product A was obtained by extraction, drying, concentration, and column chromatography (pure petroleum ether).
[0033] 1 H NMR (600MHz, CDCl 3 ,SiMe 4 ): δ=7.53(t, J=7.74Hz, 2H), 7.41(t, J=7.38Hz, 1H), 7.29(d, J=7.86Hz, 2H), 6.66-6.65(m, 2H), 6.59 (t, J=7.68Hz, 2H), 6.54-6.52 (m, 2H), 5.87 (d, J=7.98Hz, 2H).
[0034] 13 C NMR (150MHz, CDCl 3 ,SiMe 4 ): δ=143.85, 138.90, 134.33, 130.95, 130.72, 128.38, 123.15, 121.19, 115.32, 113.16.
Embodiment 2
[0036] The preparation method of the phenoxazine compound B of following structural formula:
[0037]
[0038] Into a dry 25mL Schlenk reaction tube was added the dihalide compound 2,2’-dibromodiphenyl ether (1.2mmol), followed by p-phenylenediamine) (0.6mmol), Pd(OAc) 2 (0.06mmol, 10mol%), DPEphos (0.12mmol, 20mol%), sodium tert-butoxide (3.6mmol), under the protection of nitrogen, add toluene (2mL), and react at 120°C for 72h. After the reaction, the target product B was obtained by extraction, drying, concentration, and column chromatography (petroleum ether: ethyl acetate = 20:1, volume ratio).
Embodiment 3
[0040] The preparation method of the phenoxazine compound C of following structural formula:
[0041]
[0042] To a dry 25 mL Schlenk reaction tube was added the dihalide 2-bromo-1-(2-bromophenoxy-4-chloro)-benzene (0.2 mmol), followed by aniline (0.22 mmol), Pd(OAc) 2 (0.01mmol, 5mol%), DPEphos (0.02mmol, 10mol%), sodium tert-butoxide (0.6mmol), under the protection of nitrogen, add toluene (2mL), and react at 120°C for 15h. After the reaction, the target product C was obtained by extraction, drying, concentration, and column chromatography (pure petroleum ether).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com