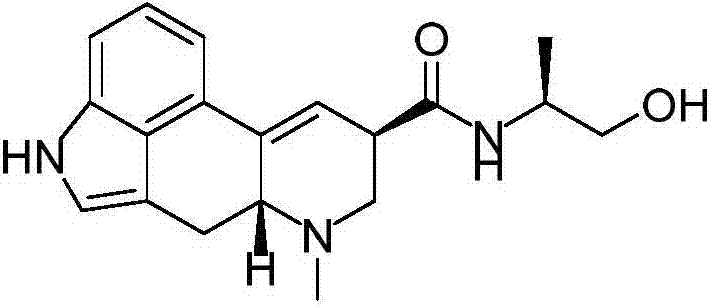
Ergometrine preparation method
A technology of ergonovine and lysergic acid, which is applied in the direction of organic chemistry, can solve the problems of low yield, and achieve the effects of high yield, mild reaction conditions, and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]Accurately weigh lysergic acid (10g, 37.3mmol), L-aminopropanol (2.8~7g, 37.3~93.25mmol), add 150mLTHF and stir, then slowly add triethylamine (7.5~18.75g, 74.6~186.5mmol), T3P (50%wt ethyl acetate solution, 23.7~71.1g, 37.3~111.9mmol). The above reaction mixture was stirred at room temperature for 6 hours, diluted with water (300mL), the organic phase was separated, the aqueous phase was extracted with dichloromethane (150mL x 2), and the combined organic phases were washed twice with saturated sodium chloride solution, 50mL each time . Fully dried with anhydrous sodium sulfate, concentrated to dryness to obtain an off-white solid, this solid was beaten with dichloromethane (50mL) to obtain 9.8g ergonovine, which was a white solid with a yield of 81%, and the optical purity was analyzed by HPLC. 98.6%.
Embodiment 2
[0035] Accurately weigh lysergic acid (10g, 37.3mmol), L-aminopropanol (2.8g, 37.3mmol), add 150mL THF and stir, then slowly add N,N-diisopropylethylamine ( 9.6 g, 74.6 mmol), T3P (50% wt in ethyl acetate, 23.7 g, 37.3 mmol). The above reaction mixture was stirred at room temperature for 6 hours, diluted with water (300mL), the organic phase was separated, the aqueous phase was extracted with ethyl acetate (150mL x 2), and the combined organic phases were washed twice with saturated sodium chloride solution, 50mL each time . Fully dried with anhydrous sodium sulfate, concentrated to dryness to obtain an off-white solid, and this solid was beaten with dichloromethane (50mL) to obtain 10.8g ergonovine, which was a white solid with a yield of 89.3%, and the optical purity was analyzed by HPLC. 99.4%.
Embodiment 3
[0037] Accurately weigh lysergic acid (10g, 37.3mmol), L-aminopropanol (2.8g, 37.3mmol), add 150mL THF and stir, then slowly add pyridine (5.9g, 74.6mmol), T3P ( 50% wt ethyl acetate solution, 23.7 g, 37.3 mmol). The above reaction mixture was stirred at room temperature for 6 hours, diluted with water (300 mL), the organic phase was separated, the aqueous phase was extracted with methyl tetrahydrofuran (150 mL x 2), and the combined organic phases were washed twice with saturated sodium chloride solution, 50 mL each time. Fully dried with anhydrous sodium sulfate, concentrated to dryness to obtain an off-white solid, this solid was beaten with dichloromethane (50mL) to obtain 10g of ergonovine, which was a white solid with a yield of 82.6%, and an optical purity of 98.9% by HPLC analysis. %.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com