Positron emitting tracer, preparation method an applicationthereof
A reaction and compound technology, which is applied in the field of medicine and its preparation, can solve problems such as interference with quantitative analysis of PET images, molecular structure instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] This embodiment provides a kind of compound [ 18 F] FDPA has the following structure:
[0108]
[0109] Chemical name: N, N-diethyl-2-(2-(4-(fluoro18-fluorophenyl))-5,7 dimethylpyrazol[1,5-a]pyrimidine)-3- base) acetamide
[0110] Molecular formula: C 20 h 23 18 FN 4 o
[0111] Molecular weight: 353.43
[0112] This compound can be used as a positron drug targeting the translocation protein TSPO.
Embodiment 2
[0114] This embodiment provides [ 18 F] the preparation method (manual preparation) of FDPA comprises the steps:
[0115] Step 1: Preparation of bare ring prosthetic group SPIAd
[0116] Add 5.0 g of malonic acid (CAS: 141-82-2, product number: A11526-100g) to 4.8 mL of acetic anhydride (Chengdu Huaxia reagent, CAS108-24-7, product number H1133817-500 mL), followed by 24 μL of concentrated sulfuric acid , the temperature of the reaction system was raised to 60° C. for 15 minutes and then cooled to room temperature.
[0117] Slowly add the above reaction system to 48mmol 2-adamantanone (Alfa Aesar, CAS 700-58-3, product number: A14275-100g), and the addition can be completed within about half an hour to one hour, and then evaporated to dryness under pressure Solvent, to obtain the bare ring prosthetic group SPIAd initial product, the bare ring prosthetic group SPIAd initial product was dissolved in 300mL anhydrous ether, backwashed with water three times, each 200mL, the orga...
Embodiment 3
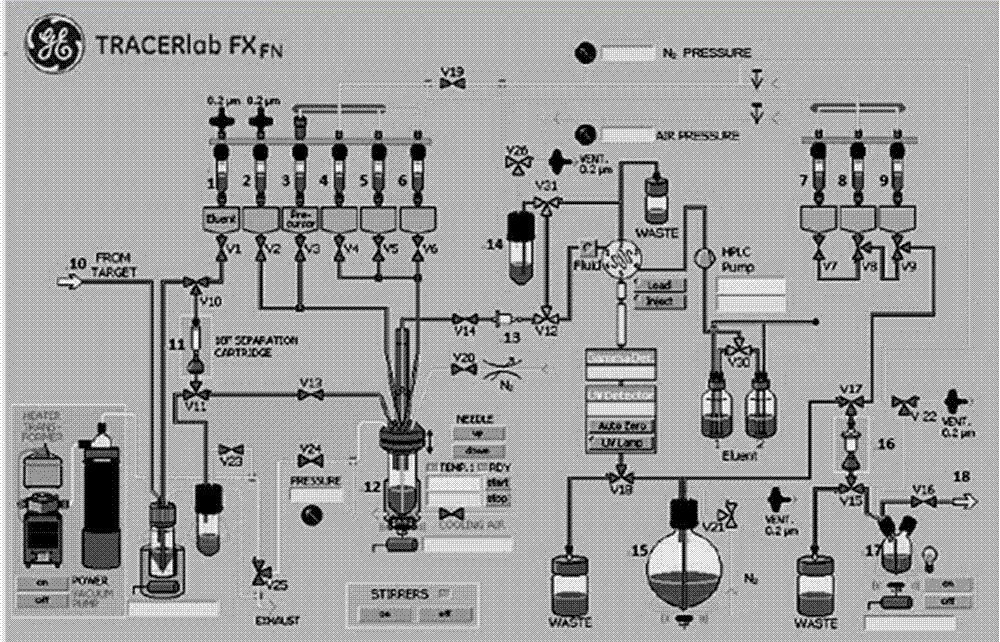
[0157] This example provides [ 18 F] FDPA synthesis system, such as figure 1 shown, including:
[0158] Fluorine-18 negative ion generator;
[0159] Fluorine-18 negative ion eluting device;
[0160] feeding device;
[0161] reaction device;
[0162] HPLC purification device;
[0163] Extraction and collection devices;
[0164] And valve v1, valve v3, valve v5, valve v6, valve v7, valve v8, valve v10, valve v13, valve v18, valve v20, valve v24, and valve v25.
[0165] The fluorine-18 negative ion generating device includes a GE cyclotron and a helium generator, and the GE cyclotron is connected to the QMA solid-phase extraction column of the eluting device through the helium generator through the valve v10,
[0166]The fluorine-18 negative ion eluting device includes an eluent generator and a QMA solid-phase extraction column, the eluent generator is a vial 1, and the vial 1 is connected up and down with the QMA solid-phase extraction column through valves v1 and v10 in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com