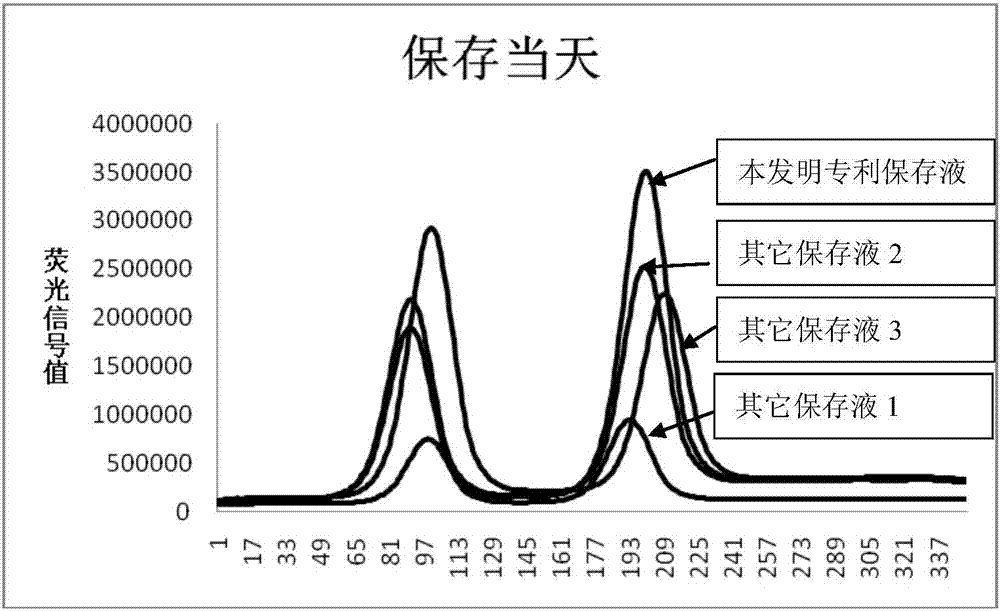
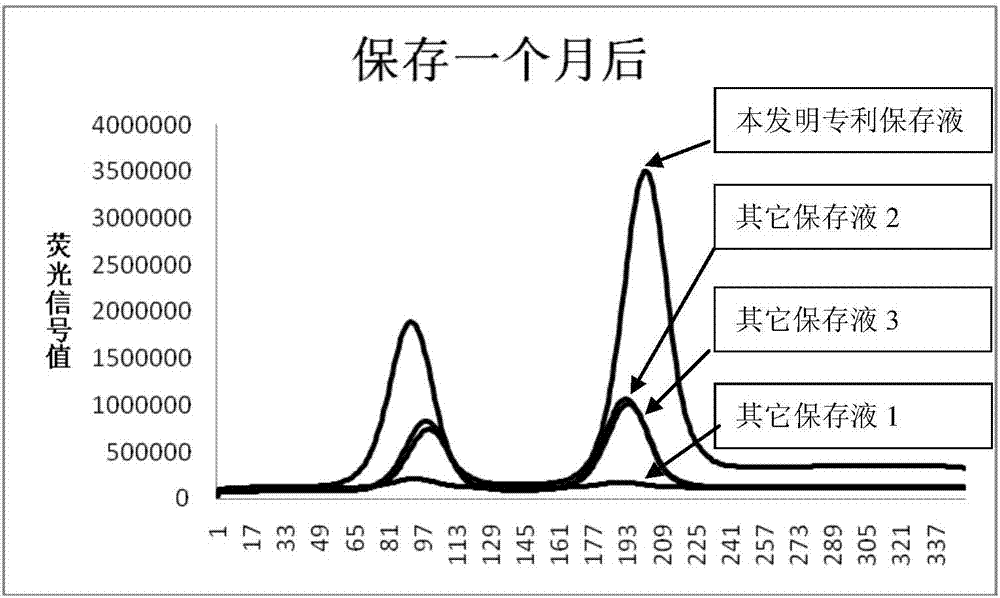
Preservation solution of time-resolved fluorescent microsphere labeled myoglobin antibody
A time-resolved fluorescence and myoglobin technology is applied in the field of time-resolved fluorescent microsphere-labeled myoglobin antibody preservation solutions, which can solve the problems of reagent detection risks, failure to see, and inability to perform detection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A time-resolved fluorescent microsphere-labeled myoglobin antibody preservation solution is prepared by the following steps:
[0027] First weigh 1.5g of dextran, dissolve it in 500g of aqueous solution, stir at 60°C for 1h, then ultrasonically for 10min, after cooling to room temperature, weigh 6g of 4-hydroxyethylpiperazineethanesulfonic acid (HEPES), chlorine Sodium Chloride (NaCl) 10g, Potassium Chloride (KCL) 5g, Disodium Ethylenediaminetetraacetic Acid (EDTA-2Na) 0.9g, Sodium Dodecyl Sulfonate (SDS) 0.3g, Bovine Serum Albumin (BSA) 10g, Triton X-100 0.5g, Tween-200.2g, Procline 0.5g, water to make up to 1000g, mix well, adjust pH to 8.5, and obtain time-resolved fluorescent microsphere-labeled myoglobin antibody preservation solution.
Embodiment 2
[0029] A time-resolved fluorescent microsphere-labeled myoglobin antibody preservation solution is prepared by the following steps:
[0030] First weigh 1g of dextran, dissolve it in 500g of aqueous solution, stir at 60°C for 1h, then sonicate for 10min, after cooling to room temperature, weigh 8g of 4-hydroxyethylpiperazineethanesulfonic acid (HEPES), chlorinated Sodium (NaCl) 14g, potassium chloride (KCL) 5g, ethylenediaminetetraacetic acid disodium (EDTA-2Na) 0.8g, sodium dodecylsulfonate (SDS) 0.5g, bovine serum albumin (BSA) 15g , Triton X-100 0.8g, Tween-20 0.2g, Procline 0.5g, make up to 1000g with water, and mix well to obtain time-resolved fluorescent microsphere-labeled myoglobin antibody preservation solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com