Preparation method of tin dioxide-titanium dioxide semiconductor-coupled ion-contra-doping photocatalytic nano fiber material
A nanofiber and semiconductor technology, applied in the field of photocatalysis, can solve the problems of limitation and high recombination rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, Sn 4+ / TiO 2 ~0.03 % preparation
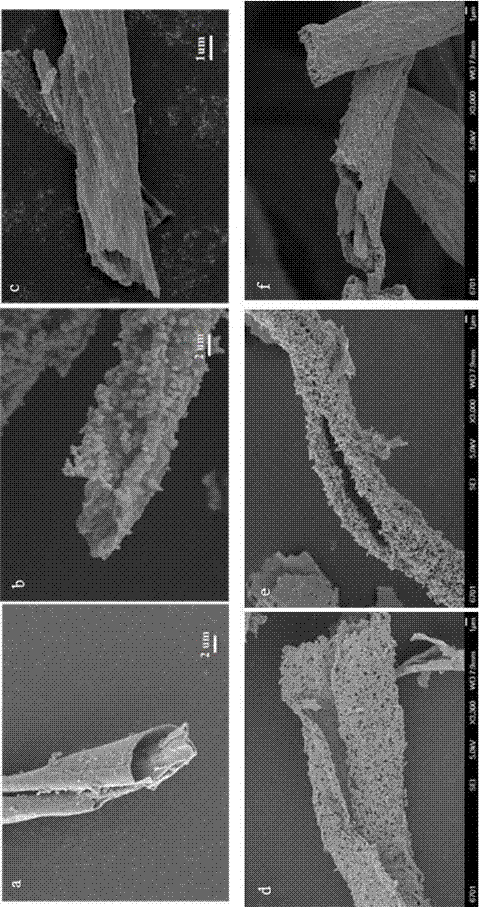
[0035] Under the condition of magnetic stirring, 1.5000 g of Ti(OC 4 h 9 ) 4 , to get A solution Ti(OC 4 h 9 ) 4 / EtOH; in A solution according to Sn 4+ The content of the substance is 0.03% by adding SnCl 4 ·5H 2 O, get mixed solution: (SnCl 4 +Ti(OC 4 h 9 ) 4 ) / EtOH; Soak 1.2000 g of CF in the mixed solution for 30 min to make Sn 4+ 、Ti 4+ Adsorbed on the surface of CF; take it out and dry it naturally in the air to obtain the precursor material (Sn 4+ +Ti 4+ ) / CF; the precursor material (Sn 4+ +Ti 4+ ) / CF was calcined at 600 ℃ for 120 min, and cooled naturally to room temperature to obtain Sn 4+ Ion-doped TiO 2 Hollow fiber structural material Sn 4+ / TiO 2 ~0.03%.
Embodiment 2
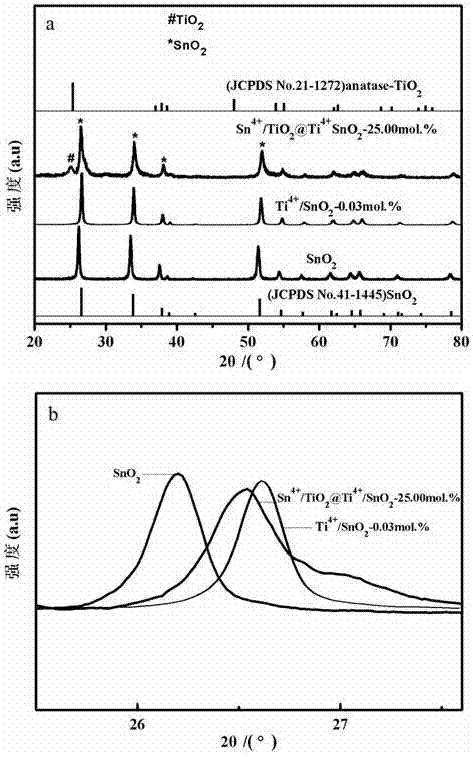
[0038] Example 2, Ti4+ / SnO 2 ~0.03% preparation
[0039] Under the condition of magnetic stirring, add 1.5000 g of SnCl to 75.00 mL of EtOH 4 ·5H 2 O, get B solution SnCl 4 / EtOH; then in solution B according to Ti 4+ The content of the substance is 0.03% by adding Ti(OC 4 h 9 ) 4 , to get a mixed solution (Ti(OC 4 h 9 ) 4 +SnCl 4 ) / EtOH; Soak 1.2000 g of CF in the mixed solution for 30 min to make Sn 4+ 、Ti 4+ The ions are adsorbed on the surface of CF, taken out and dried naturally in the air to obtain the precursor material (Sn 4+ +Ti 4+ ) / CF; the precursor material (Sn 4+ +Ti 4+ ) / CF was calcined at 600 ℃ for 120 min, and cooled naturally to room temperature to obtain Ti 4+ Ion-doped SnO 2 Hollow fiber structural material Ti 4+ / SnO 2 ~0.03%.
[0040] Comparative example: pure SnO 2 Preparation: Add 1.5000 g of SnCl to 75.00 mL of EtOH under magnetic stirring 4 ·5H 2 O, get B solution SnCl 4 / EtOH; Soak 1.2000 g of CF in solution B for 30 min to m...
Embodiment 3
[0042] Embodiment 3, ion anti-doping, semiconductor coupling material Ti 4+ / SnO 2 @Sn 4+ / TiO 2 preparation of
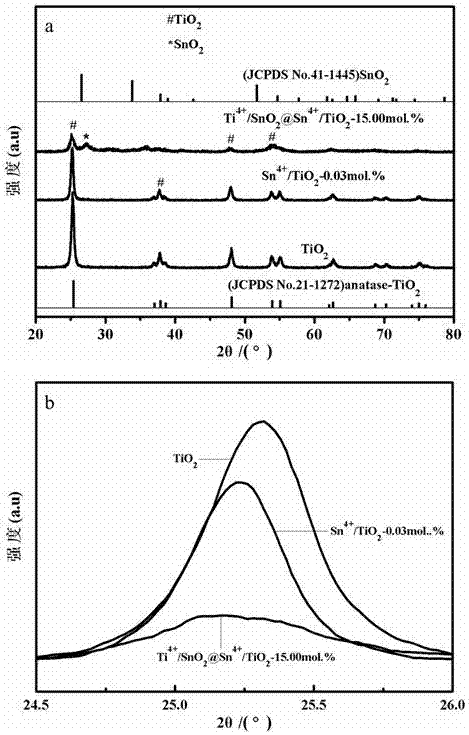
[0043] Under the condition of magnetic stirring, 1.5000 g of Ti(OC 4 h 9 ) 4 , to get A solution Ti(OC 4 h 9 ) 4 / EtOH; in A solution according to SnO 2 The content of the substance is 15.00% by adding SnCl 4 ·5H 2 O, get mixed solution: (SnCl 4 +Ti(OC 4 h 9 ) 4 ) / EtOH; Soak 1.2000 g of CF in the mixed solution for 30 min to make Sn 4+ 、Ti 4+ Adsorbed on the surface of CF; take it out and dry it naturally in the air to obtain the precursor material (Sn 4+ +Ti 4+ ) / CF; the precursor material (Sn 4+ +Ti 4+ ) / CF was calcined at 600 ℃ for 120 min, and cooled naturally to room temperature to obtain TiO 2 Ion counterdoping as the host phase, TiO 2 and SnO 2 Coupling Hollow Fiber Structural Material Ti 4+ / SnO 2 @Sn 4+ / TiO 2 ~15.00 %.
[0044] with pure TiO 2 Compared to that in sample Ti 4+ / SnO 2 @Sn 4+ / TiO 2 ~15.00 % in XRD, the main ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com