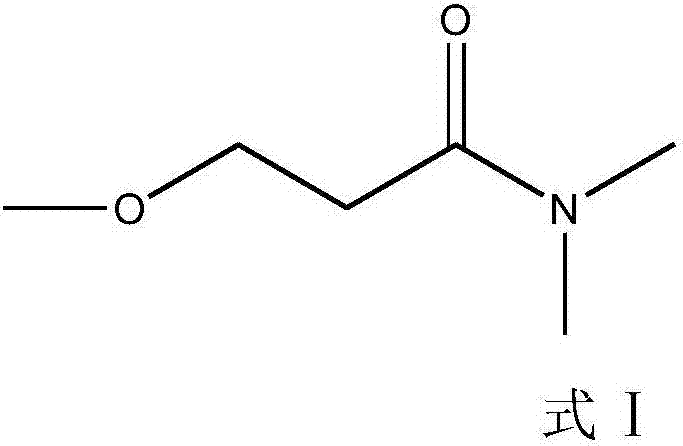
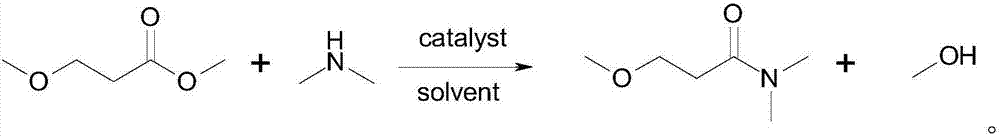
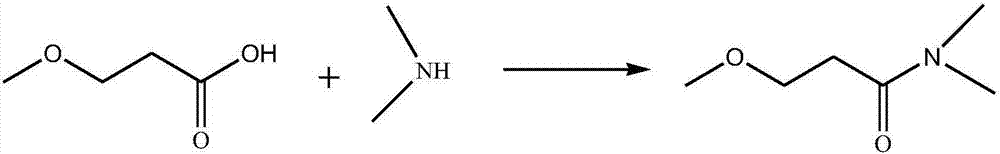Synthesis method of 3-methoxyl-N, N-dimethylacrylamide
A dimethylpropionamide and synthesis method technology, applied in the synthesis of 3-methoxy-N,N-dimethylpropionamide, and in the field of synthesis of chemical material intermediates, can solve difficult processing, high energy consumption, The process is cumbersome and other problems, to achieve the effect of simple reaction, low energy consumption and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037] The following are specific examples of the present invention to further describe the technical solutions of the present invention, but the present invention is not limited to these examples.
[0038] According to the parameters in Table 1 Examples 1-38 and Comparative Examples 3-20 and adopt the following method of the present invention to synthesize 3-methoxy-N,N-dimethylpropionamide:
[0039] S1. Synthesis of 3-methoxypropionitrile: Add acrylonitrile, anhydrous methanol and metal alkoxides to the reactor for alkoxylation first, then recover unreacted anhydrous methanol to obtain 3-methoxypropionitrile propionitrile;
[0040] Synthesis of S2, 3-methoxypropionic acid: adding acidic catalyst and water to 3-methoxypropionitrile in step S1, heating for hydrolysis reaction to obtain 3-methoxypropionic acid;
[0041] S3. Synthesis of 3-methoxy-N,N-dimethylpropionamide: Add 3-methoxypropanoic acid and dimethylamine into a closed reactor, heat up and react to obtain 3-methoxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com