Amino acid polypyridyl copper complex and preparation method and application thereof
A technology of copper complexes and amino acids, applied in copper organic compounds, pharmaceutical formulations, organic chemical methods, etc., can solve the problems of limited clinical wide application, toxic and side effects, severe drug resistance, etc., and achieve good anticancer activity and preparation method. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
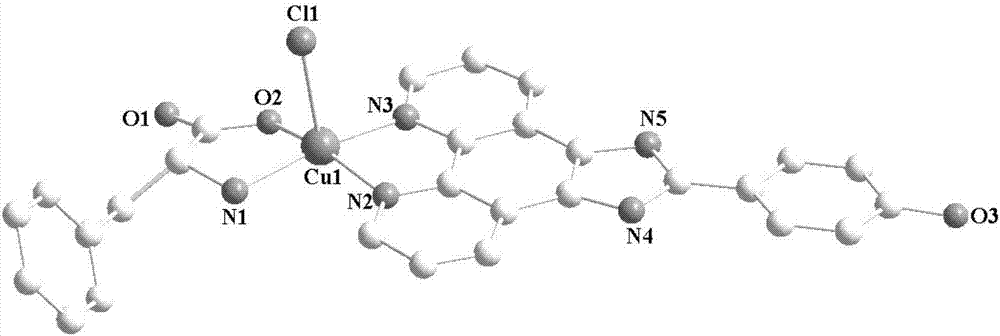
[0031] Synthesis of copper complex 1:
[0032] Dissolve 0.5mmol of amino acid and 0.5mmol of alkali in 20mL of water and 20mL of alcohol mixed solution, add 0.5mmol of copper chloride under stirring at room temperature, reflux and stir for 3 hours, filter, add 0.5mmol of ammonium hexafluorophosphate, and continue the reaction at room temperature After 1 hour, filter, and the filtrate evaporates slowly at room temperature. After 3 weeks, green crystals suitable for X-ray analysis are precipitated, and the yield is about 48% (calculated as copper salt). Elemental analysis results (%), experimental value: C, 54.80; H, 4.62; N, 11.40. Theoretical value (C 28 h 28 N 5 o 5 ClCu): C, 54.81; H, 4.60; N, 11.41, the results are basically consistent with the theoretical value.
Embodiment 2
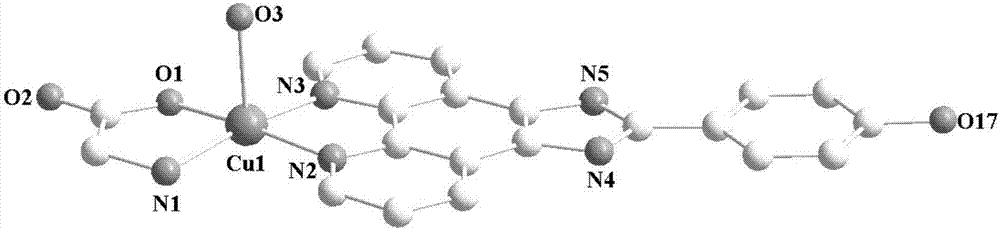
[0034] Synthesis of copper complex 2:
[0035] Dissolve 0.5mmol of amino acid and 0.5mmol of base in 20mL of 1 water and 20mL of alcohol mixed solution, add 0.5mmol of copper nitrate under stirring at room temperature, reflux and stir for 3 hours, filter, add 0.5mmol of ammonium hexafluorophosphate, and continue the reaction at room temperature for 1 hour, filtered, and the filtrate was slowly volatilized at room temperature. After 3 weeks, a green crystal suitable for X-ray analysis was separated out, and the yield was about 41% (calculated as copper salt), elemental analysis result (%), experimental value: C, 44.54; H, 3.95; N, 14.85. Theoretical value (C 21 h 22 N 6 o 9 Cu): C, 44.56; H, 3.92; N, 14.85. The results are basically consistent with the theoretical value.
Embodiment 3
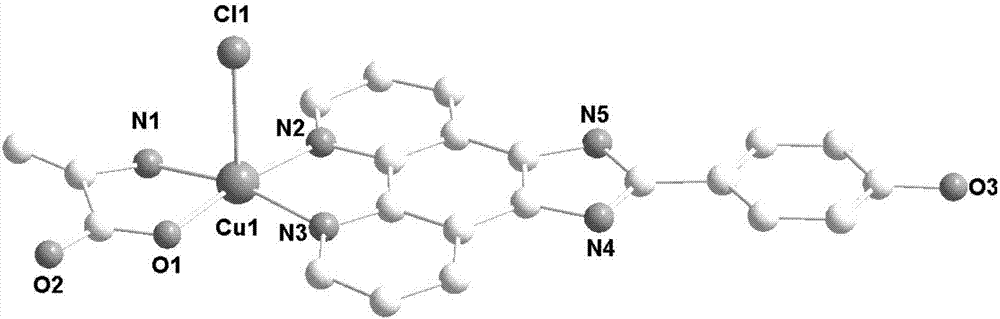
[0037] Synthesis of copper complex 3:
[0038] Dissolve 0.5mmol of amino acid and 0.5mmol of alkali in 20mL of water and 20mL of alcohol mixed solution, add 0.5mmol of copper chloride under stirring at room temperature, reflux and stir for 3 hours, filter, add 0.5mmol of ammonium hexafluorophosphate, and continue the reaction at room temperature After 1 hour, filter, and the filtrate evaporates slowly at room temperature. After 3 weeks, green crystals suitable for X-ray analysis are precipitated, and the yield is about 43% (calculated as copper salt). Elemental analysis results (%), experimental value: C, 52.90; H, 3.65; N, 14.01. Theoretical value (C 22 h 18 N 5 o 3 ClCu): C, 52.91; H, 3.63; N, 14.02, the results are basically consistent with the theoretical value.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com