Kit and application for detecting urinary pathogenic bacteria
A urine disease and reagent kit technology, applied in the field of medical testing, can solve problems such as high cost, high technical requirements, and low throughput detection, and achieve the effects of simple operation, high detection sensitivity, and shortened detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
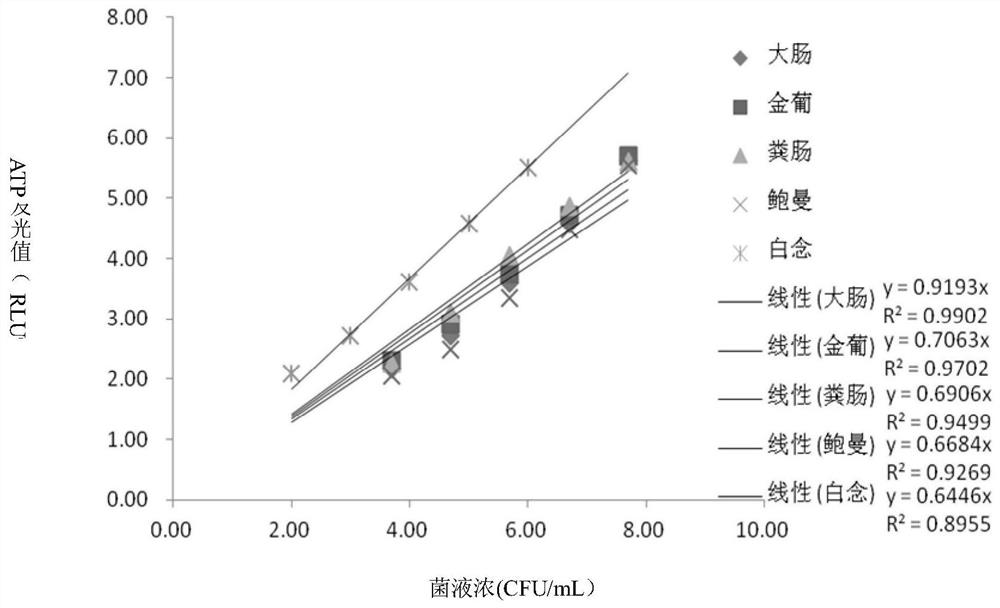
[0042] Embodiment 1: the standard curve of pathogenic bacteria of urine sample.
[0043] Test strains: as follows (Table 1), purchased from Guangdong Microbial Culture Collection Center.
[0044] Table 1 Standard strains
[0045]
[0046] Urine source: The midsection urine of several healthy volunteers was collected and mixed for use.
[0047]Urine pathogenic bacteria ATP detection kit includes urine pretreatment reagents, pathogenic bacteria ATP lysate, neutralizing solution, and ATP detection reagents. The urine pretreatment reagent is composed of somatic cell digestion solution and ATPase, the composition of somatic cell digestion solution is 0.3-0.7% Triton and 0.03-0.07% TCA, the ATPase is 95-105U / mL APYrase; the ATP lysate of pathogenic bacteria is 2-5% TCA; the neutralizing solution is composed of 100-123.4mmol / LTris-base, 2-2.34mmol / L magnesium acetate, and the pH is adjusted to 8.2; the ATP detection reagent is composed of luciferase, luciferin and buffer, The c...
Embodiment 2
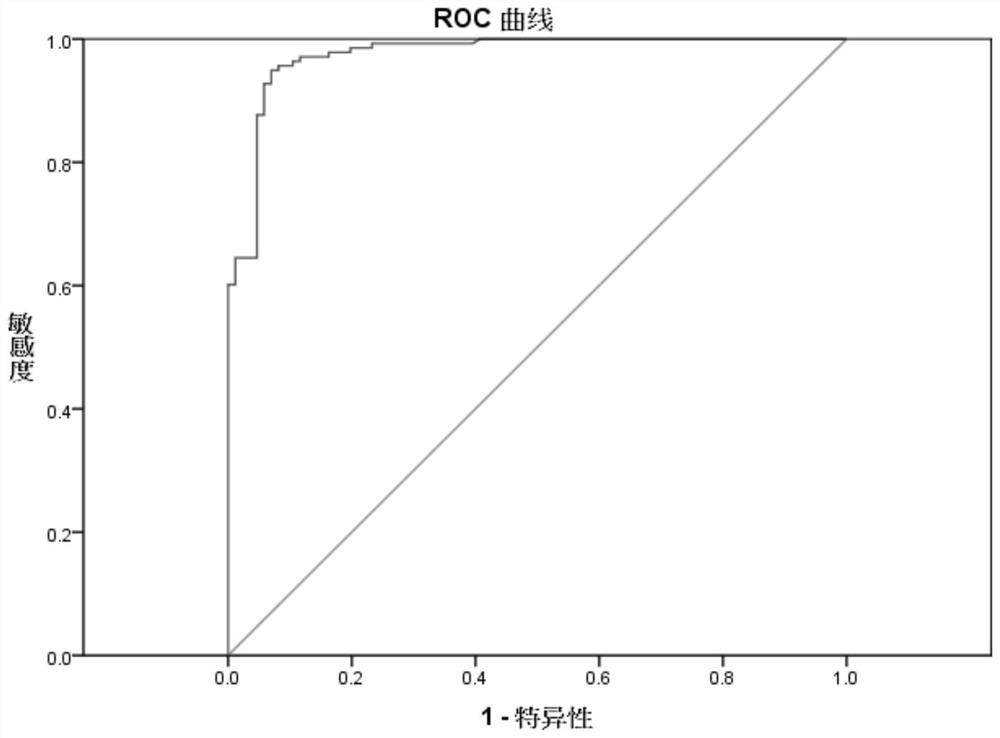
[0058] Example 2: Determination of the critical value for the detection of pathogenic bacteria ATP in urine samples.
[0059] Urine specimen collection: 224 cases of urine specimens were collected from the Department of Microbiology of our hospital.
[0060] Urine pathogenic bacteria ATP detection kit includes urine pretreatment reagents, pathogenic bacteria ATP lysate, neutralizing solution, and ATP detection reagents. The urine pretreatment reagent is composed of somatic cell digestion solution and ATPase, the composition of somatic cell digestion solution is 0.5% Triton by volume fraction and 0.05% TCA by mass fraction, and APYrase with 100U / mL ATPase; The mass fraction is 4% TCA; the neutralizing solution is composed of 123.4mmol / L Tris-base, 2.34mmol / L magnesium acetate, and the pH is adjusted to 8.2; the ATP detection reagent is composed of luciferase, luciferin and buffer solution, of which The concentration of luciferase was 16 μg / mL, the concentration of luciferin wa...
Embodiment 3
[0071] Example 3: Detection of ATP of pathogenic bacteria in clinical urine samples.
[0072] Urine specimen collection: 1055 urine specimens were collected from three tertiary hospitals.
[0073] Urine pathogenic bacteria ATP detection kit includes urine pretreatment reagents, pathogenic bacteria ATP lysate, neutralizing solution, and ATP detection reagents. The urine pretreatment reagent is composed of somatic cell digestion solution and ATPase, the composition of somatic cell digestion solution is 0.5% Triton by volume fraction and 0.05% TCA by mass fraction, and APYrase with 100U / mL ATPase; 4% TCA; the neutralizing solution is composed of 123.4mmol / L Tris-base, 2.34mmol / L magnesium acetate, and the pH is adjusted to 8.2; the ATP detection reagent is composed of luciferase, luciferin and buffer solution, of which luciferase The concentration was 16 μg / mL, the concentration of fluorescein was 20 μg / mL, the buffer was composed of 20 mmol / L Tris-base and 2 mmol / L magnesium ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com