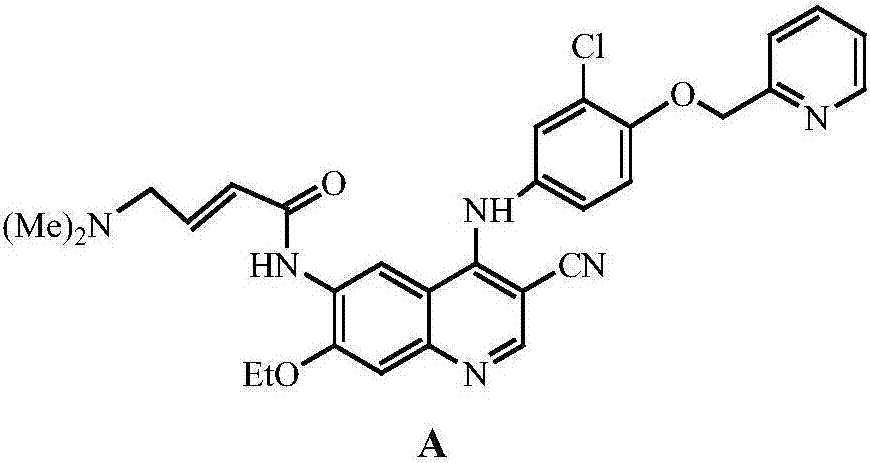
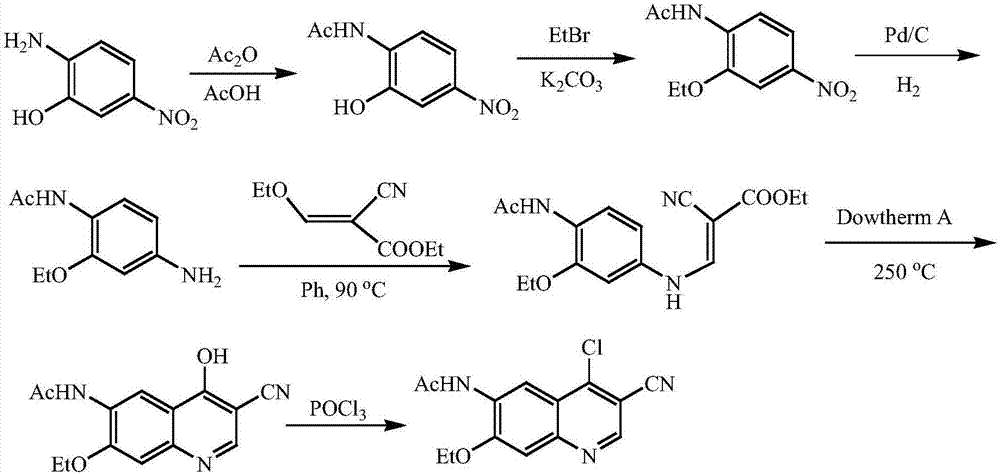
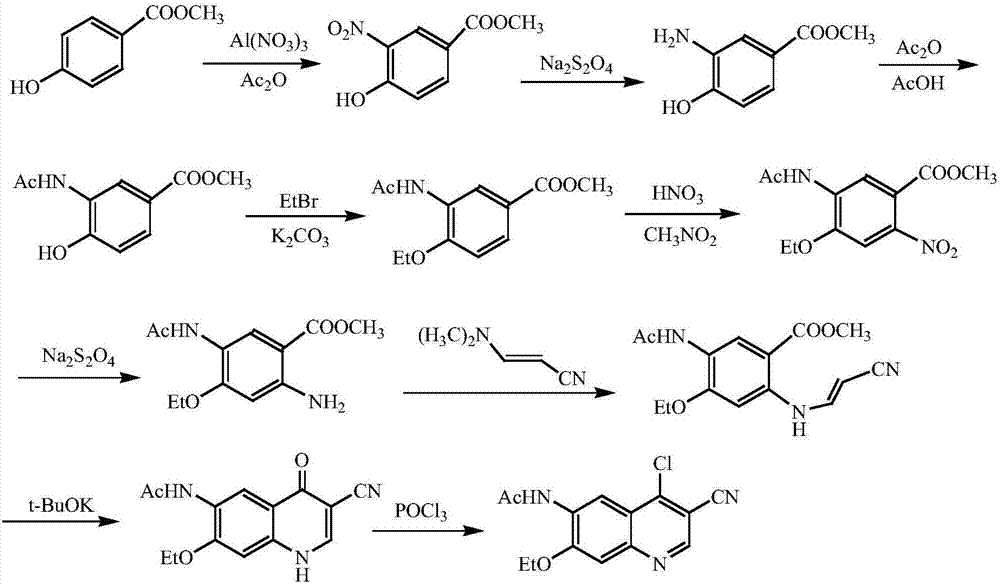
A method of synthesizing a neratinib intermediate, 3-cyano-4-chloro-6-amino-7-ethoxyquinoline
A technology of ethoxyquinoline and neratinib, which is applied in the direction of organic chemistry, can solve the problems of complex operation, poor process safety, and difficult availability of raw materials, and achieve simple operation, high total yield, and cheap reagents Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Preparation of compound of formula (III) 2-(4-ethoxy-2-chloro-5-nitrobenzoyl)-3-aminoacrylonitrile
[0026] Add 50mmol 3-aminoacrylonitrile, 50mmol solid base catalyst ZrO 2 -Cr 2 O 3 Add 50mL of tetrahydrofuran and 50mL of tetrahydrofuran to the reaction flask, stir evenly at room temperature, and then drop a mixture of 55mmol of methyl 4-ethoxy-2-chloro-5-nitrobenzoate and 15mL of tetrahydrofuran into the above reaction flask. The reaction was stirred for 2 hours, and then refluxed for 2 hours. After cooling, the catalyst was filtered, and the catalyst could be reused after drying. The solvent was evaporated under reduced pressure, 400 mL of dichloromethane was added to the residue, washed with 50 mL of distilled water for three times, the organic layers were combined, dried with anhydrous sodium sulfate, and then the solvent was evaporated under reduced pressure to obtain the compound of formula (III) with a yield of 87 %.
Embodiment 2
[0027] Example 2 Preparation of compound of formula (IV) 3-cyano-4-oxo-6-nitro-7-ethoxy-1,4-dihydroquinoline
[0028] Add 40mmol of formula (III) compound, 40mmol of anhydrous potassium carbonate and 40mL of DMF into the reaction flask, stir the reaction at 50~60℃ for 4h, cool to room temperature, add 60mL water, stir for 0.5h, filter the precipitated solid, The solid was recrystallized with ethanol and dried under reduced pressure to obtain the compound of formula (IV) with a yield of 90%.
Embodiment 3
[0029] Example 3 Preparation of compound 3-cyano-4-chloro-6-nitro-7-ethoxyquinoline of formula (V)
[0030] Add 40 mmol of the compound of formula (IV) and 180 mL of phosphorus oxychloride to the reaction flask, and heat and stir to reflux for 3 hours. Cool the reaction flask to about 0°C, and slowly pour 1500mL 2mol / L sodium carbonate solution into the reaction flask at this temperature, stir for 0.5h, filter with suction, wash the filter cake with warm water, and dry under reduced pressure to obtain the formula (V ) Compound, the yield is 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com