Prenylated flavonoid, and applications thereof in preparing drugs used for treating inflammatory diseases
A technology for isopentenyl flavonoids and inflammatory diseases, which is applied to the application field of isopentenyl flavonoids in the preparation of medicines for treating inflammatory diseases, and can solve damage to vascular endothelial cells and extravascular tissue cells, tissue damage, Problems such as blocking microcirculation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
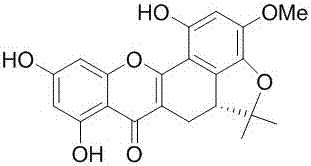
[0018] Example 1 Preparation of prenyl flavone artoheteroid C in jackfruit
[0019] The jackfruit root medicinal material (17 Kg) was extracted by leakage with 95% ethanol, and the extract was concentrated under reduced pressure to obtain 1.5 Kg of extract. The extract was suspended in water, extracted successively with petroleum ether, chloroform, ethyl acetate and n-butanol, and concentrated to dryness respectively. Take 532 g of the extract from the chloroform extraction part, perform macroporous resin column chromatography (column size: 15*55cm, 5.3 Kg), and elute with ethanol-water gradient to obtain 10 fractions frs. H1-H10. Fraction H5 (10.0 g) was applied to a Sephadex LH-20 column and eluted with methanol to obtain 10 fractions: frs.H5L1-H5L10. Fraction H5L5 (1.5 g) was applied to a reverse ODS column and eluted with methanol-water (2:8→10:0, v / v) to obtain 4 fractions H5L5O1-H5L5O4. Fraction H5L5O4 (483.0 mg) was further applied to Sephadex LH-20 gel column, eluted...
Embodiment 2
[0020] Example 2 Structural Identification of the Prenyl Flavone Artoheteroid C in Jackfruit
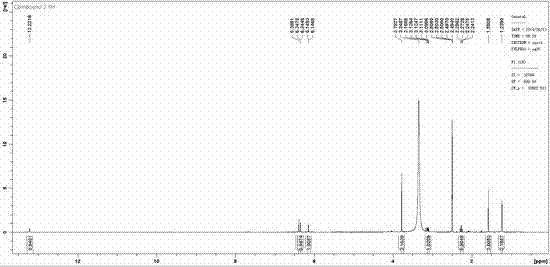
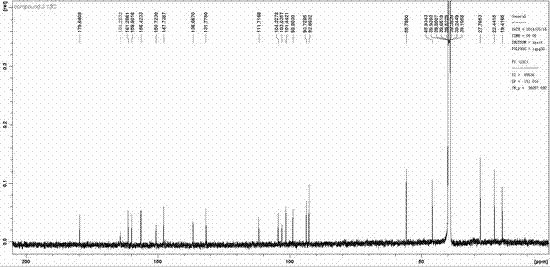
[0021] Artoheteroid C, a yellow amorphous powder with quasi-molecular ion peaks by HR-ESI-MS m / z 381.0979 ([M-H] - , C 21 h 17 o 7 , Calculated value: 381.0980), confirm its molecular formula as C 21 h 18 o 7 . IR ( ν max 3430, 2974, 1651, 1612, 1504, 1467, 1356 cm -1 ) and UV spectra ( lambda max 263, 316, 378 nm) showed that the compound had typical absorption characteristics of prenylflavonoids. Artoheteroid C's 1 H NMR spectrum (600 MHz, dimethyl sulfoxide- d 6 ) shows the following proton signal: a hydrogen-bonded hydroxyl δ H 12.33(1H, s, OH-5); a pair of meta-coupled aromatic protons δ H 6.33 (1H, d, J = 1.8 Hz, H-8) and 6.13(1H, d, J = 1.8 Hz, H-6); a lone aromatic proton δ H 6.38 (H-3'); a methoxy group δ H 3.77(3H, s, MeO-4′); two methyl groups δ H 1.57 (3H, s, H 3 -14) and 1.23 (3H, s, H 3 -15) and an ABX-type spin-coupled system δ ...
Embodiment 3
[0025] Example 3 Cytotoxicity evaluation experiment of artoheteroid C on rat PMNs
[0026] The cytotoxicity of artoheteroid C on PMNs was determined by referring to the relevant literature of the standard trypan blue exclusion method. At 37°C, 1 mL of PMNs cell suspension (1×10 6 ) (2% FCS-HBSS) were incubated with 10 μL DMSO or artoheteroid C (final concentration ranging from 1 to 1000 μM) for 30 min. 112 μL of 0.4% trypan blue staining solution was added to each sample, and under a high-power microscope, the cytotoxic effect of the sample on PMNs was calculated by counting the absorption of trypan blue by 100 cells. The results showed that artoheteroid C at 180 M above began to show toxicity to PMNs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com