Establishing method of fingerprint of traditional Chinese medicinal composition preparation
A technology of fingerprint spectrum and establishment method, which can be used in measurement devices, instruments, scientific instruments, etc., and can solve the problems of inability to fully characterize the chemical characteristics and quality of Zhuangyao Jianshen Pills.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Example 1 Establishment of the method for preparing the test solution
[0135] 1.1 Selection of extraction solvent:
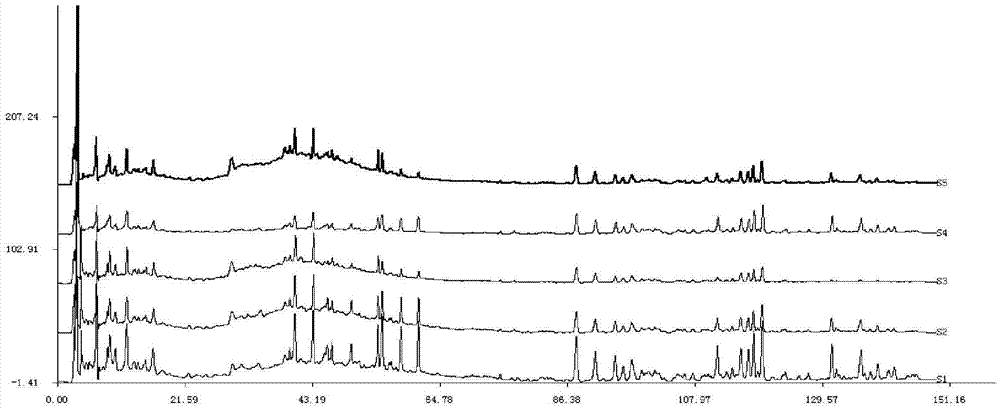
[0136] Get the Zhuangyaojianshen pill sample 5g of batch number C08047, grind finely, accurately weigh, place in the Erlenmeyer flask with stopper of 50ml, add 25ml methyl alcohol, 75% methanol (volume percent concentration), 50% methanol (volume percent concentration) respectively accurately percent concentration), 95% ethanol (volume percent concentration), 50% ethanol (volume percent concentration), after ultrasonication for 45 minutes, let cool to room temperature, weigh, and use extraction solvent to supplement the lost weight respectively, shake well , filter, and take the continued filtrate, that is, too.
[0137] Under the following chromatographic conditions, measure the test solution prepared by different extraction solvents respectively, and record the chromatogram of 0-150min:
[0138] Column: Phenomenex Luna 5uC 18 (2) 100A chromatograp...
Embodiment 2
[0152] Example 2 Establishment of Chromatographic Conditions
[0153] 2.1 Determination of mobile phase
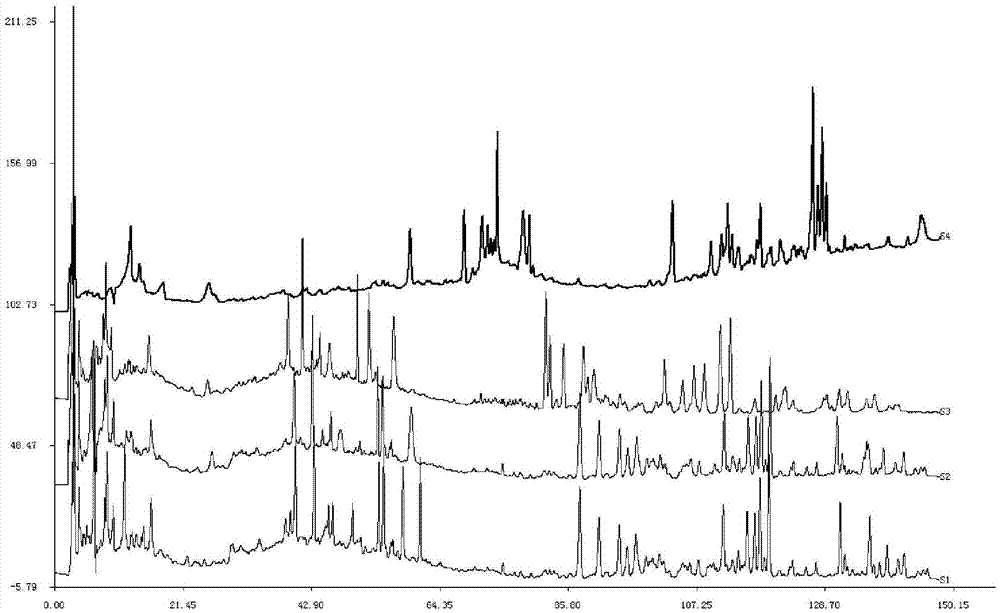
[0154] According to the preferred preparation method of the test solution in Example 1, about 5 g of the sample of batch number C08047 was taken to prepare the test solution. The column temperature is 30°C, the injection volume is 10 μl, the flow rate is 1ml / min, and the detection wavelength is 230nm. ), acetonitrile (A)-methanol (B)-water (C) and acetonitrile (A)-methanol (B)-0.1% phosphoric acid aqueous solution (C) are mobile phases, gradient elution, elution procedure is shown in Table 1. The elution time is 150min, and the chromatogram is recorded. Chromatogram see figure 2 .
[0155] The results showed that acetonitrile-methanol-0.1% phosphoric acid aqueous solution was the mobile phase, the baseline was relatively stable and the separation effect was the best. Therefore, acetonitrile-methanol-0.1% phosphoric acid aqueous solution was selected as the mobile p...
Embodiment 3
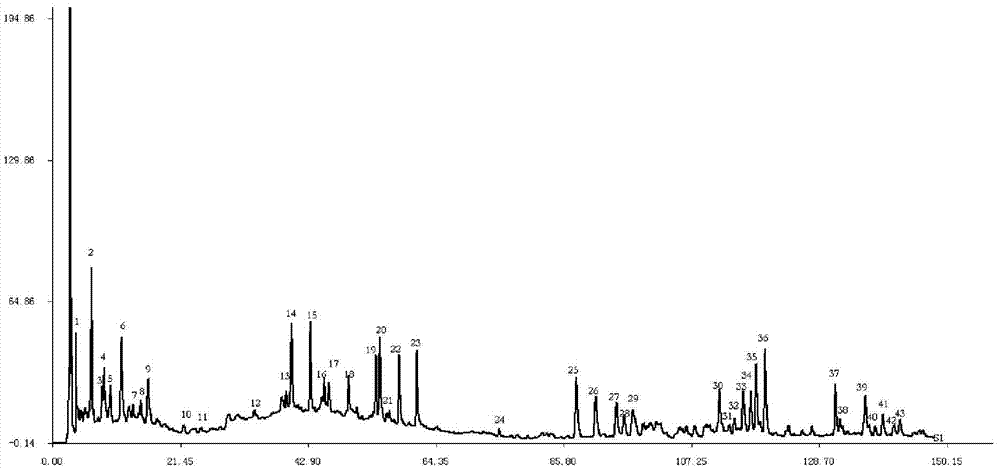
[0160] Example 3 Composition testing of Zhuangyaojianshen pills
[0161] 1. Column: Phenomenex Luna 5u C 18 (2) 100A chromatographic column (250×4.6mm, 5μm);
[0162] 2. Mobile phase: Acetonitrile-methanol-0.1% phosphoric acid aqueous solution is the mobile phase, and the gradient elution program is shown in Table 1;
[0163] 3. Flow rate: 1.0ml / min;
[0164] 4. Detection wavelength: 230nm;
[0165] 5. Column temperature: 30°C;
[0166] 6. the preparation of need testing solution: prepare according to the preferred method of embodiment 1;
[0167] 7. Preparation of reference solution: Precisely weigh protocatechuic acid, protocatechualdehyde, privetin, schizandrin A and (2′S)-schisandrin reference substance, and add methanol to prepare The concentration is a mixture of protocatechuic acid 120 μg / ml, protocatechualdehyde 130 μg / ml, privetin 140 μg / ml, Japanese Schizandrin A 40 μg / ml and (2′S)-Schisandra lignin 30 μg / ml Reference substance solution, to obtain;
[0168] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com