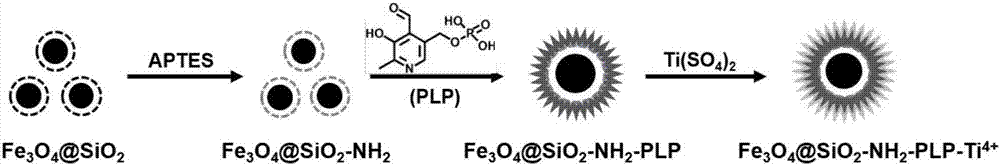
Method for preparing immobilized metal affinity material by using 5-pyridoxal phosphate
A technology of affinity materials and metals, which is applied in the field of analytical chemistry, can solve the problems of complex synthesis process and high toxicity, and achieve the effect of simple modification method and increased practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] First prepare amino-modified nanomaterials to obtain Y-NH 2 ; Then connect 5-pyridoxal phosphate to Y-NH 2 On, Y-NH with surface-modified phosphate2 -PLP; Finally, metal ions are immobilized on the phosphate to obtain Y-NH 2 -PLP-M n+ .
[0031] 1. Amino-modified nanomaterials (Y-NH 2 ) preparation, according to different nanomaterials, its preparation method is as follows:
[0032] ① SiO 2 -NH 2 Preparation: 0.5g nano-SiO 2 Disperse in 25mL of 1M HCl aqueous solution for activation for 30min; after washing with water and ethanol, disperse it in 50mL of ethanol; add 2mLAPTES to the above suspension, stir at room temperature for 3h to obtain amino-bonded silica gel nanoparticles.
[0033] ② Fe 3 o 4 @SiO 2 -NH 2 Preparation: 2.0g FeCl 2 4H 2 O and 5.4 g FeCl 3 ·6H 2 O was dissolved in 2M HCl aqueous solution, nitrogen gas was passed through to remove dissolved oxygen, then 30mL concentrated ammonia water (25wt%) was added and stirred for 30min to obtain Fe...
Embodiment 2
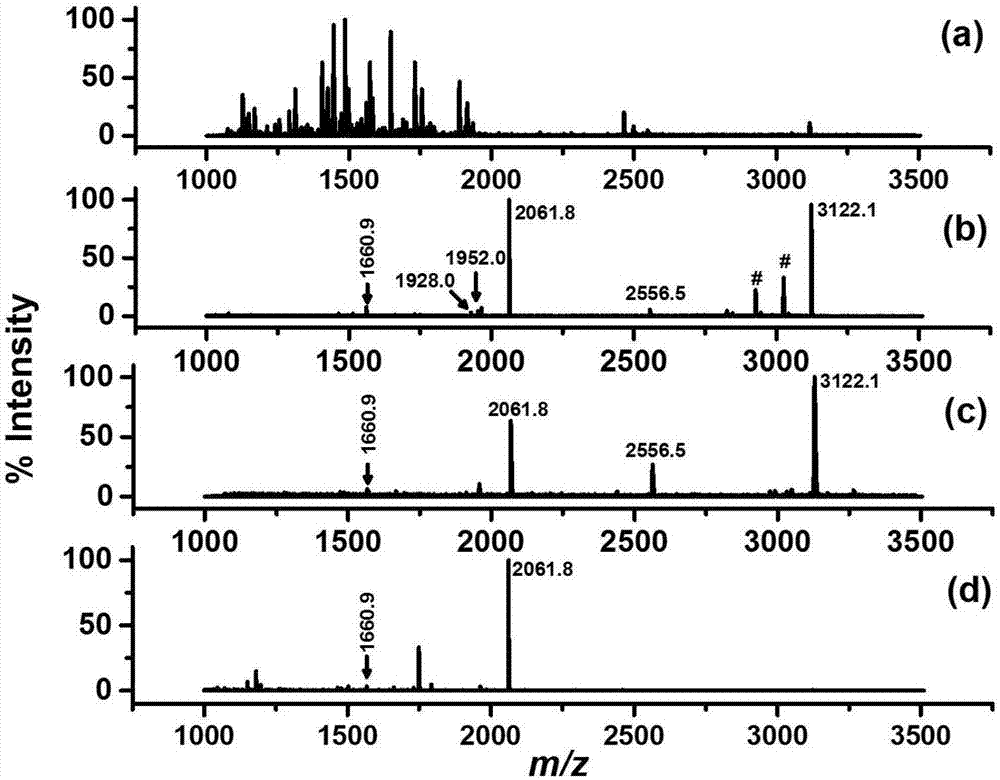
[0039] In order to investigate three different nanomaterials modified titanium ions (Y-NH 2 -PLP-Ti 4+ ) on the extraction effect of phosphorylated peptides, so as to select an optimal substrate material, we compared three kinds of Y-NH 2 -PLP-Ti 4+ (Y=SiO 2 , OCNT or Fe 3 o 4 @SiO 2 ) Extraction effect on phosphorylated polypeptide in β-casein and BSA enzymatic hydrolysis mixture:
[0040] Prepare β-casein with 100mM Tris-HCl (pH=8.5) buffer solution to a concentration of 1mg / mL; add pancreatic protease, and reacted at 37°C for 16h. The polypeptide product after enzymatic hydrolysis was drained by a vacuum centrifugal concentrator and stored in a -20°C refrigerator for later use.
[0041] Weigh 1 mg BSA and dissolve it in 100 μL of denaturing buffer (pH=8.5) containing 8M urea and 100 mM Tris-HCl, and then add 5 μL of 100 mM tris(2-formylethyl)phosphine hydrochloride solution (tricarboxymethylphosphoric acid, TCEP), and react at room temperature for 20 min to reduce ...
Embodiment 3
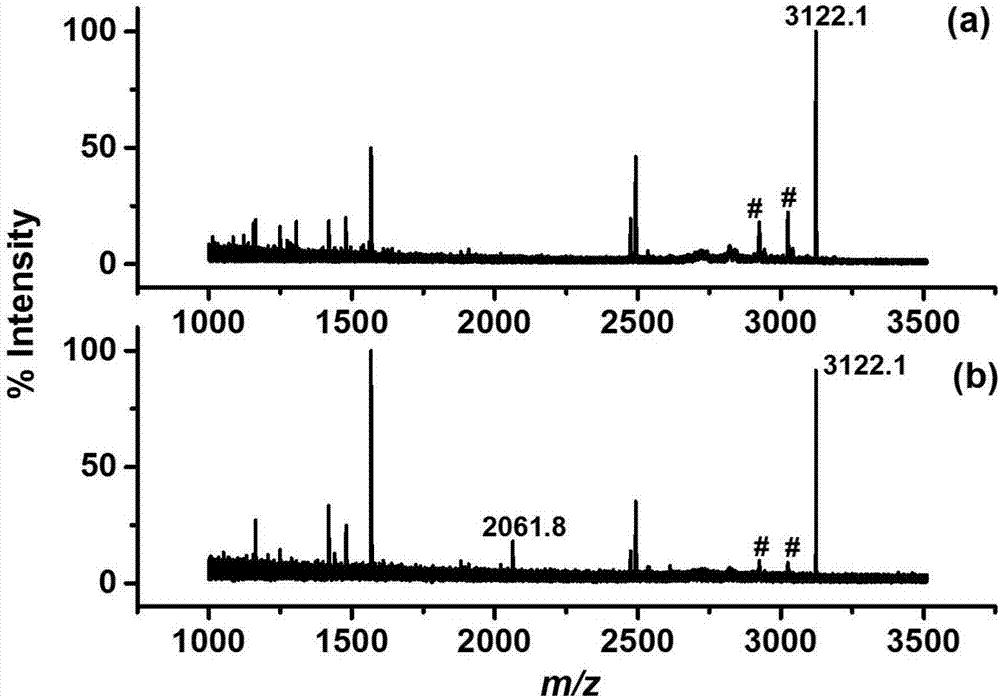
[0047] In order to investigate three commonly used metal ions (Ti 4+ , Ga 3+ , Fe 3+ ) on the extraction effect of phosphorylated peptides, so as to select an optimal metal ion to obtain the optimal material, we compared the Fe 3 o 4 @SiO 2 -NH 2 -PLP-M n+ (M=Ti 4+ , Ga 3+ or Fe 3+ ) Extraction effect on phosphorylated polypeptide in β-casein and BSA enzymatic hydrolysis mixture:
[0048] In order to compare three Fe bound to different metal ions 3 o 4 @SiO 2 -NH 2 -PLP-M n+ (M=Ti 4+ , Ga 3+ or Fe 3+ ) for the enrichment ability of phosphorylated peptides, the mixture of phosphorylated protein (β-casein) and non-phosphoprotein (BSA) hydrolyzate was used as the analyte (β-casein:BSA=1:500).
[0049] Weigh 10mg Fe respectively 3 o 4 @SiO 2 -NH 2 -PLP-M n+Disperse in 1mL of water, mix well, take 20μL of the dispersion, equilibrate with the sample solution (5% TFA-50% ACN (v / v)), then add it to 100μL of the sample solution containing the peptide mixture, vort...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com