Synthetic method of lipid-lowering drug ciprofibrate
A technology of blood lipid-lowering drug and synthesis method, which is applied in the field of synthesis of blood lipid-lowering drug ciprofibrate, which can solve the problems of insufficient stability of raw material styrene, uneasy properties of hydroxystyrene, and insufficient mild reaction conditions, etc., and achieve post-processing steps The effect of simplicity, strong economy, and short reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
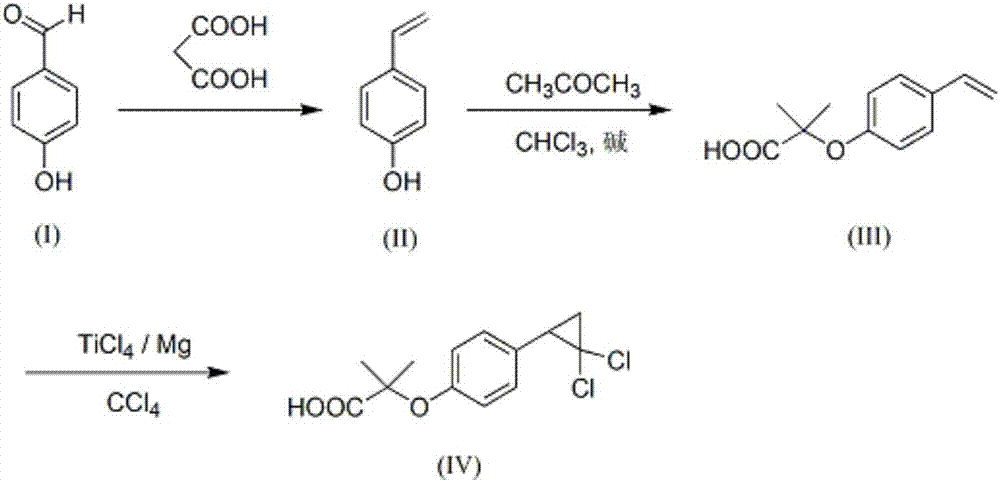
[0032] The invention provides a synthesis method of ciprofibrate. The route uses p-hydroxybenzaldehyde as a raw material to obtain a final product through Knoevenagel-decarboxylation reaction, Bargllini reaction and olefin insertion reaction. In the Knoevenagel-decarboxylation cascade reaction, a mixed solvent is used to replace the traditional single solvent, which effectively reduces the reaction time and promotes the conversion of intermediate products; in the olefin insertion reaction, TiCl4 / Mg / CCl4 is used to replace the traditional carbene addition System to build a three-membered ring, avoiding the use of a strong base, the reaction is carried out at a lower temperature, the conditions are mild, and the energy consumption is reduced. Specific steps include:
[0033] (1) p-Hydroxybenzaldehyde (I), generates p-hydroxystyrene (II) through heating and alkali catalysis with malonic acid in a mixed solvent;
[0034] (2) p-hydroxystyrene (II) and acetone, chloroform, and alkali...
Embodiment 1
[0042] (1) Synthesis of p-hydroxystyrene (II)
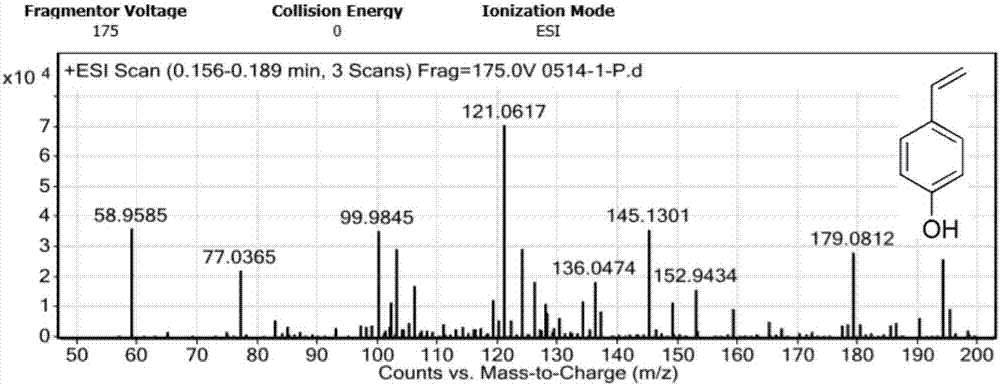
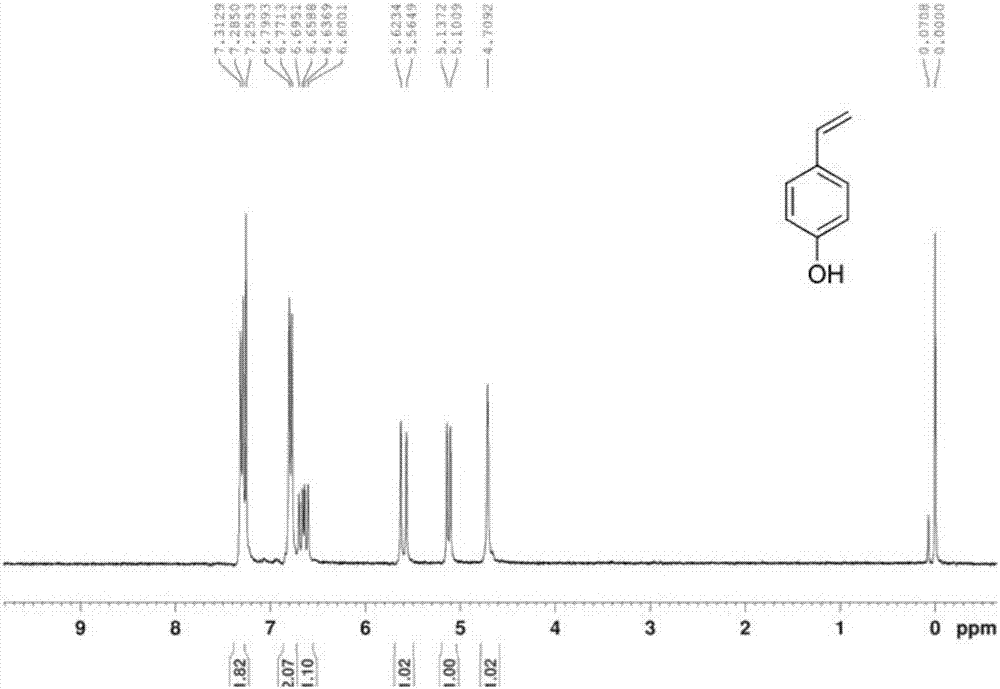
[0043] Dissolve 50g of p-hydroxybenzaldehyde (I) in 250mL of toluene, add 85g of malonic acid and 41.5mL of diethylamine, heat to reflux at 150°C, and divide the water with a water separator. After reacting for 2 hours, separate the oily product, then add 250ml of DMF, continue to heat up to 150°C, react for 2 hours, cool the reaction liquid, adjust the pH to 3-4 with 1mol / L dilute hydrochloric acid in an ice bath, and extract with ethyl acetate. Organic phase saturated with NaHCO 3 solution washing, saturated NaCl solution washing, anhydrous Na 2 SO 4 After drying, the solvent was distilled off under reduced pressure to obtain 46.0 g of crude p-hydroxystyrene (II) oil, with a yield of 94.6%.
[0044] (2) Synthesis of 2-methyl-2-(4-vinylphenoxy)propionic acid (III)
[0045] Dissolve 10g of p-hydroxystyrene (II) in 70mL of acetone, add 9.9g of NaOH and 2.6g of tetra-n-butylammonium bromide, and heat at 40°C to make it reflux s...
Embodiment 2
[0050] (1) Synthesis of p-hydroxystyrene (II)
[0051] Dissolve 50g of p-hydroxybenzaldehyde (I) in 500mL of 1:1 (v / v) DMF / toluene, add 85g of malonic acid and 41.5mL of diethylamine, heat to reflux at 150°C, and divide the water with a water separator. After reacting for 4 hours, the reaction solution was cooled, and the pH was adjusted to 3-4 with 1mol / L dilute hydrochloric acid in an ice bath, extracted with ethyl acetate, and the organic phase was washed with saturated NaHCO 3 solution washing, saturated NaCl solution washing, anhydrous Na 2 SO 4 After drying, the solvent was distilled off under reduced pressure to obtain 46.0 g of the crude p-hydroxystyrene (II) oil, with a yield of 93.5%.
[0052] (2) Synthesis of 2-methyl-2-(4-vinylphenoxy)propionic acid (III)
[0053] Dissolve 10g of p-hydroxystyrene (II) in 70mL of acetone, add 9.9g of NaOH and 2.6g of tetra-n-butylammonium bromide, and heat at 40°C to make it reflux stably; dissolve 29.8g of chloroform in about 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com