Preparation method of dodecanoyl-beta cyclodextrin and preparation method of polysulfone membrane for tryptophan chiral resolution
A technology of dodecanoyl and cyclodextrin, applied in chemical instruments and methods, membrane technology, semipermeable membrane separation, etc., can solve the problems of cumbersome preparation, high cost, and high toxicity of tryptophan chiral separation membranes, etc. Achieve the effects of low preparation cost, good stability and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
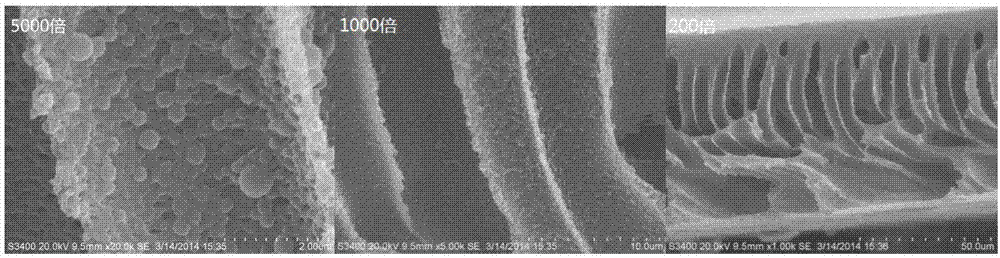
Image
Examples
Embodiment 1
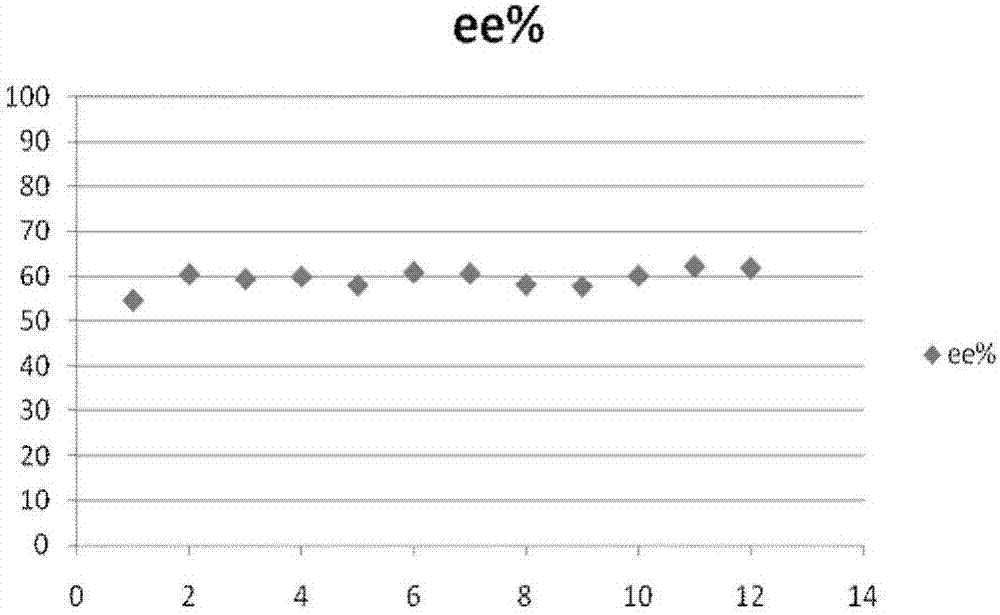
[0033] First prepare the lauryl-β-cyclodextrin polysulfone membrane by the following steps, and then evaluate the concentration ratio of the two configurations in the filtrate filtered by the chiral membrane by a validated HPLC method to resolve chiral substances Split performance.
[0034] Synthesis of dodecanoyl-β-cyclodextrin:
[0035] Dry recrystallized β-cyclodextrin (5.675 g, 5 mmol) was slowly added to dry pyridine (100 mL) under magnetic stirring. After the β-cyclodextrin was completely dissolved, a certain mass of lauryl chloride (M β-环糊精 :M 十二酰氯 =1:7). The above mixed solution was shaken and reacted for 65 h at room temperature. After the reaction, the reaction liquid was evaporated to dryness in a rotary evaporator at 70° C. under reduced pressure to obtain a crude product of the reaction. Add 100 mL of water to the reaction crude product, stir magnetically at 100° C. for 10 min, and filter to obtain the reaction product. Dry the reaction product in a vacuum o...
Embodiment 2
[0049] The preparation method of lauryl-beta-cyclodextrin comprises the following steps:
[0050] (1) Add dry recrystallized β-cyclodextrin (3g, 2.64mmol) into anhydrous pyridine (50mL);
[0051] (2) After the β-cyclodextrin is completely dissolved, add lauryl chloride dropwise, (M β-环糊精 :M 十二酰氯 =1:6);
[0052] (3) The above mixed solution was shaken and reacted for 40 hours at room temperature;
[0053] (4) After the reaction, the reaction solution was evaporated to dryness at 60°C under reduced pressure to obtain the crude reaction product
[0054] (5) Add 50 mL of water to the reaction crude product, stir at 100°C for 20 minutes, filter to obtain the reaction product, and vacuum-dry the reaction product at 15°C to constant weight to obtain dodecanoyl-β-cyclodextrin.
[0055] The preparation method of lauryl-beta-cyclodextrin polysulfone chiral membrane comprises the following steps:
[0056] (1) Under stirring conditions, heat and stir 10% (7.5% PSF+2.5% lauryl-β-cyclo...
Embodiment 3
[0062] The preparation method of lauryl-beta-cyclodextrin comprises the following steps:
[0063] (1) Add dry recrystallized β-cyclodextrin (8g, 7mmol) into anhydrous pyridine (100mL);
[0064] (2) After the β-cyclodextrin is completely dissolved, add lauryl chloride dropwise, (M β-环糊精 :M 十二酰氯 =1:8);
[0065] (3) The above mixed solution was shaken and reacted for 80 hours at room temperature;
[0066] (4) After the reaction, the reaction solution was evaporated to dryness at 80°C under reduced pressure to obtain the crude reaction product
[0067] (5) Add 300 mL of water to the reaction crude product, stir at 70°C for 5 minutes, and filter to obtain the reaction product, which is vacuum-dried at 50°C to constant weight to obtain dodecanoyl-β-cyclodextrin.
[0068] The preparation method of lauryl-beta-cyclodextrin polysulfone chiral membrane comprises the following steps:
[0069] (1) Under stirring conditions, heat and stir 28% (18.5% PSF+9.5% dodecanoyl-β-cyclodextrin)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com