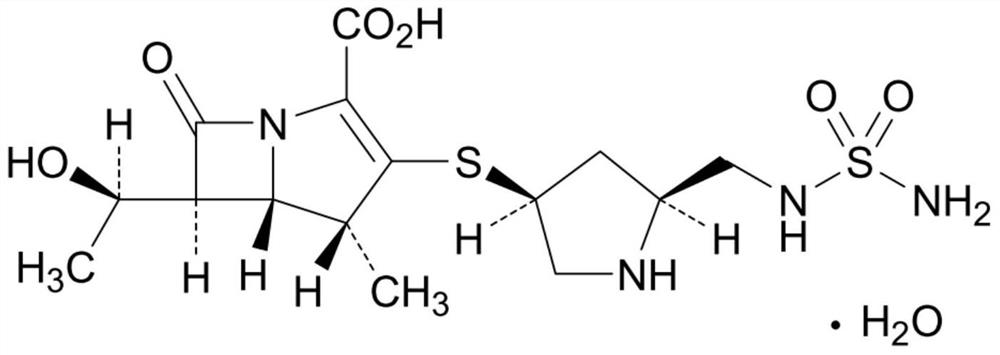
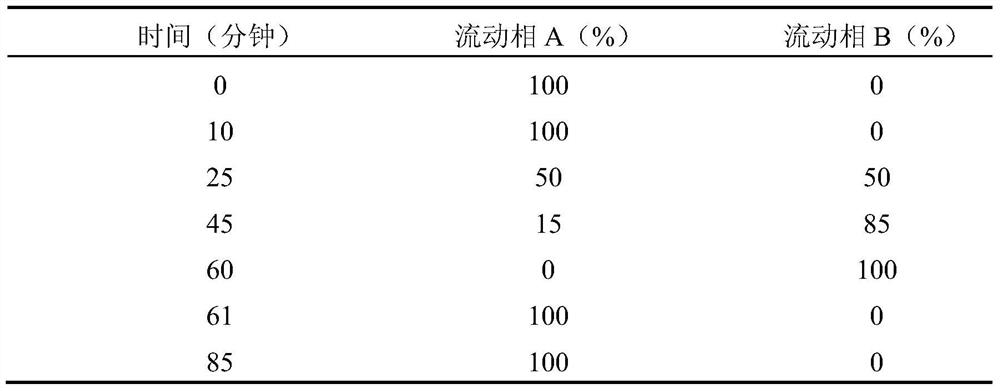
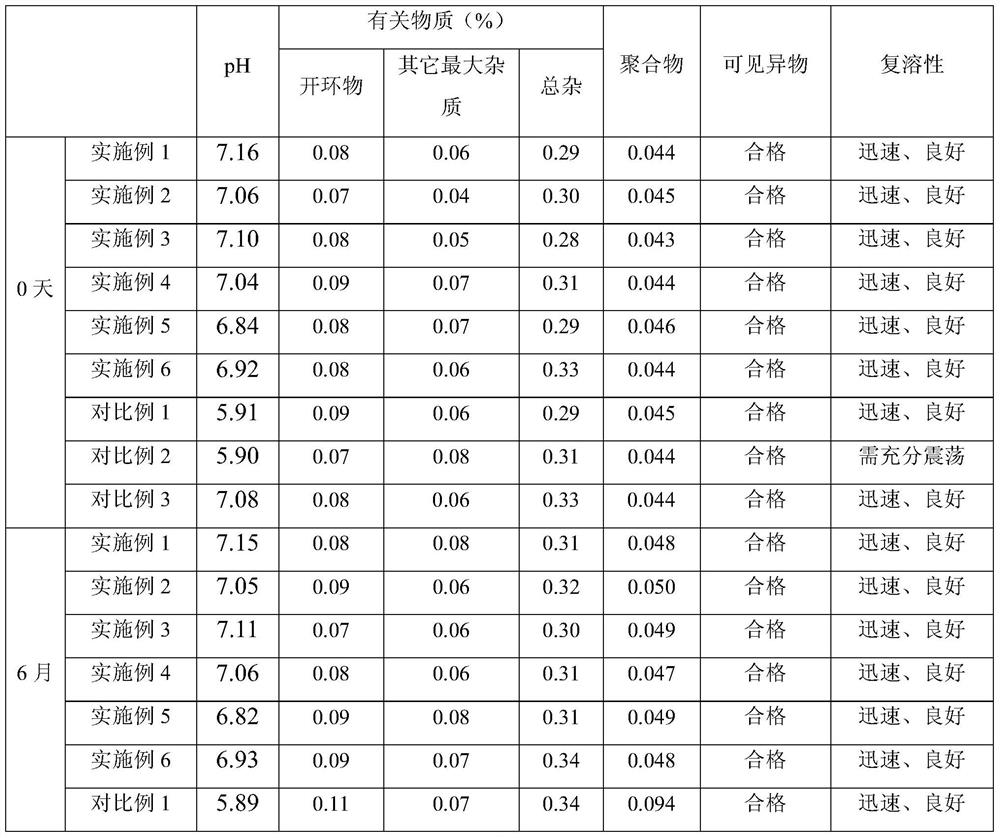
A kind of doripenem for injection and preparation method thereof
A technology for doripenem and injection, applied in the field of medicine, can solve the problems of unstable preparation, difficulty in reconstitution of doripenem and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Doripenem of 500g and sodium bicarbonate of 50g are dissolved in the mixed solvent of 50L of water and methanol (the volume ratio of water: methanol is 1:7), and the obtained solution is filtered with a 0.22um microporous membrane Sterilized and filtered, ready for use.
[0025] (2) Autoclave the spray-dried cabinet body with pure steam, the sterilization temperature is 121°C, and the sterilization time is 15 minutes.
[0026] (3) The feed liquid in (1) was heated to 45°C, fed into a spray dryer, and spray-dried at 45°C under nitrogen purging, and the drying time was 8h.
[0027] (4) Under the protection of nitrogen, the obtained granules are divided into clean vials, each bottle contains 0.25g, the bottle is filled with nitrogen, and then the rubber stopper is pressed tightly, and the aluminum cap is rolled outside.
Embodiment 2
[0029] (1) Doripenem of 500g and sodium bicarbonate of 25g are dissolved in the mixed solvent (water: the volume ratio of isopropanol is 1:27) of the water of 50L, isopropanol, gained solution is used 0.22um Microporous membrane sterilization filtration, spare.
[0030] (2) Sterilize the spray-dried cabinet body with pure steam autoclave, the sterilization temperature is 121°C, and the sterilization time is 30 minutes.
[0031] (3) The feed liquid in (1) was heated to 65°C, fed into a spray dryer, and spray-dried at 65°C under nitrogen purging, and the drying time was 10h.
[0032] (4) Under the protection of nitrogen, the obtained granules are divided into clean vials, each bottle contains 0.25g, the bottle is filled with nitrogen, and then the rubber stopper is pressed tightly, and the aluminum cap is rolled outside.
Embodiment 3
[0034] (1) Dissolve 500 g of doripenem and 37.5 g of sodium bicarbonate in a mixed solvent of 50 L of water: methanol: isopropanol (the volume ratio of water: methanol: isopropanol is 1:15:15) , the obtained solution was sterilized and filtered with a 0.22um microporous membrane for use.
[0035] (2) Sterilize the spray-dried cabinet with pure steam by autoclaving, the sterilization temperature is 121°C, and the sterilization time is 20 minutes. (3) The feed liquid in (1) was heated to 55°C, fed into a spray dryer, and spray-dried at 55°C under nitrogen purging, and the drying time was 9h.
[0036] (4) Under the protection of nitrogen, the obtained granules are divided into clean vials, each bottle contains 0.25g, the bottle is filled with nitrogen, and then the rubber stopper is pressed tightly, and the aluminum cap is rolled outside.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com