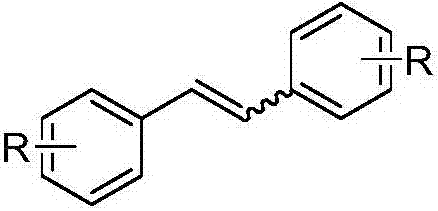
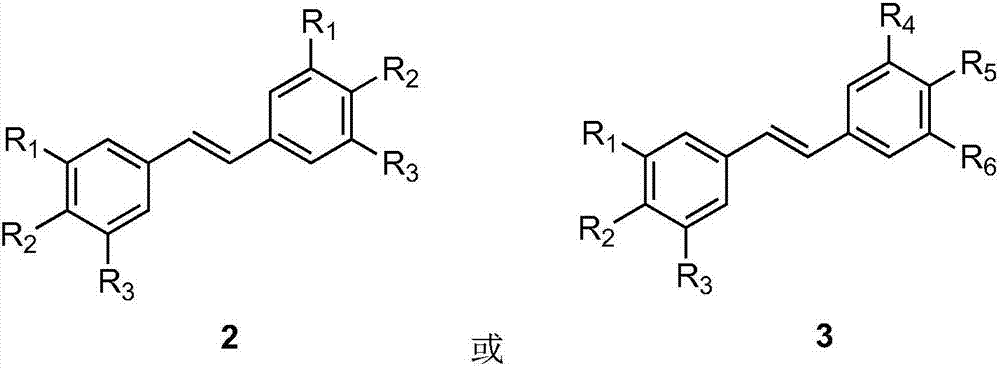
Method for preparing trans-diphenylethylene compound
A technology of stilbene and compounds, which is applied in the field of preparation of organic compounds, can solve the problems of poor compatibility of different functional groups and low yield of trans-stilbene products, and achieve variable structure, good compatibility, The effect of mild synthetic conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] (1) Preparation of raw materials
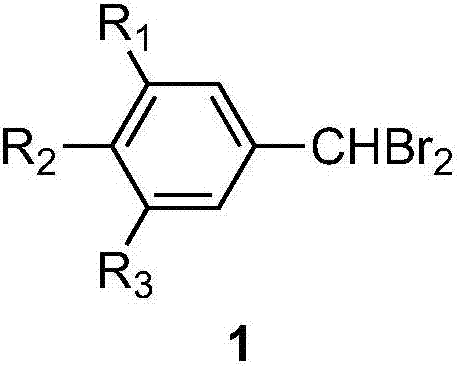
[0040] All gem-dibromomethyl arene compounds can be prepared by existing common synthesis methods, and the molecular structure of the gem-dibromomethyl arene compounds used in the examples is shown in the following formula.
[0041]
[0042] Raw compound abbreviation R 1
R 2
R 3
1a H- H- H- 1b H- CH 3 -
H- 1c H- CH 3 CH 2 -
H- 1d H- tert-C 4 h 9 -
H- 1e H- CH 3 OC(O)-
H- 1f H- CH 3 CH 2 OC(O)-
H- 1g H- CH 3 C(O)-
H- 1h H- CH 3 COO-
H- 1i H- Ph-trans-CH=CH-C(O)- H- 1j CH 3 O-
H- H- 1k H- Ph-i-C 3 h 6 -
H- 1l CH 3 O-
CH 3 O-
CH 3 O-
[0043] (2) Synthesis method
[0044] Deoxidize a certain stoichiometric ratio of gem-dibromomethyl aromatic compounds, polyamines, and copper powder (it can also be replaced with copper wire or copper sheet) in the reaction flask...
Embodiment 1
[0051] The synthesis of embodiment 1 dibromotoluene (1a)
[0052] Dissolve 3 mL of benzaldehyde (30 mmol) in 30 mL of CH 2 Cl 2 Add 30mL of 1.2M BBr slowly dropwise under stirring at 0℃ 3 CH 2 Cl 2 The solution (36 mmol) was dropped in 0.5 hours, and the temperature was raised to 25° C. to continue the reaction for 3 hours. The reaction solution was light orange, and white waxy globular solids settled out at the bottom. The reaction was stopped. After filtering, the filtrate was passed through a 200-300 mesh silica gel column, rinsed with petroleum ether, and the eluent was concentrated to obtain colorless transparent droplets. After vacuum drying at room temperature, 5.1 g of light yellow droplets were obtained, with a yield of 68%. 1 H NMR (400MHz, CDCl 3 ,ppm): δ=7.56(d,2H,ArH),7.37(m,3H,ArH),6.65(s,1H,ArCHBr 2 ).
Embodiment 2
[0053] The synthesis of embodiment 2 methyl dibromomethylbenzoate (1e)
[0054] 1.500g methyl p-toluate (10mmol), 3.920g NBS (22mmol) and 0.266g BPO (1.1mmol), 60mL CCl 4 Sequentially added to a 100mL three-necked flask, the system was ventilated with nitrogen for 15 minutes, and refluxed for 3 hours to stop the reaction. After filtration, the filtrate was concentrated to obtain a light yellow solid. The crude product was recrystallized from n-hexane to obtain 707 mg of white crystals with a yield of 23%. 1 H NMR (400MHz, CDCl 3 ,ppm): δ=8.04(d,2H,ArH),7.65(d,2H,ArH),6.66(s,1H,ArCHBr 2 ),3.93(s,3H,-OCH 3 ). EA: Calcd. For C 9 h 8 Br 2 o 2 C(%): 35.10, H(%): 2.62. Found C(%): 35.42, H(%): 2.54.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com