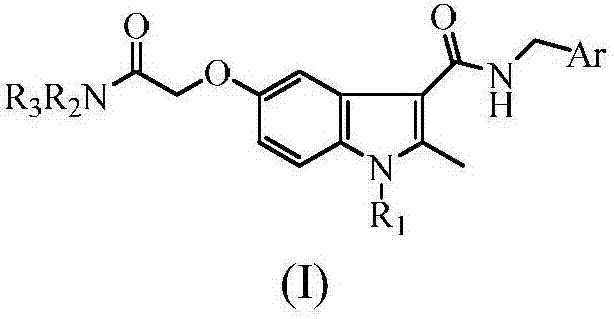
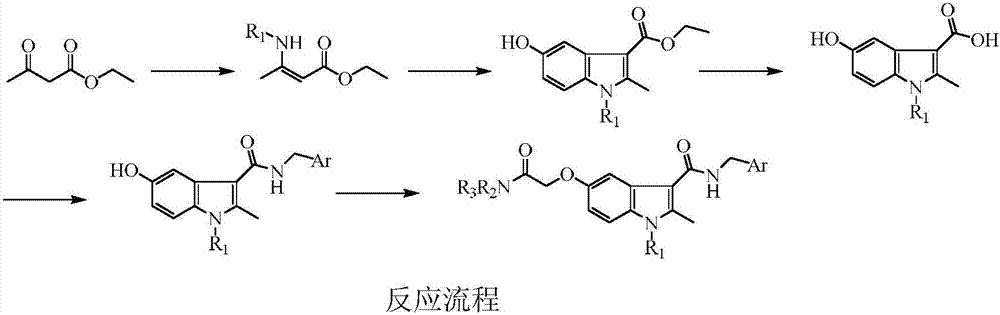
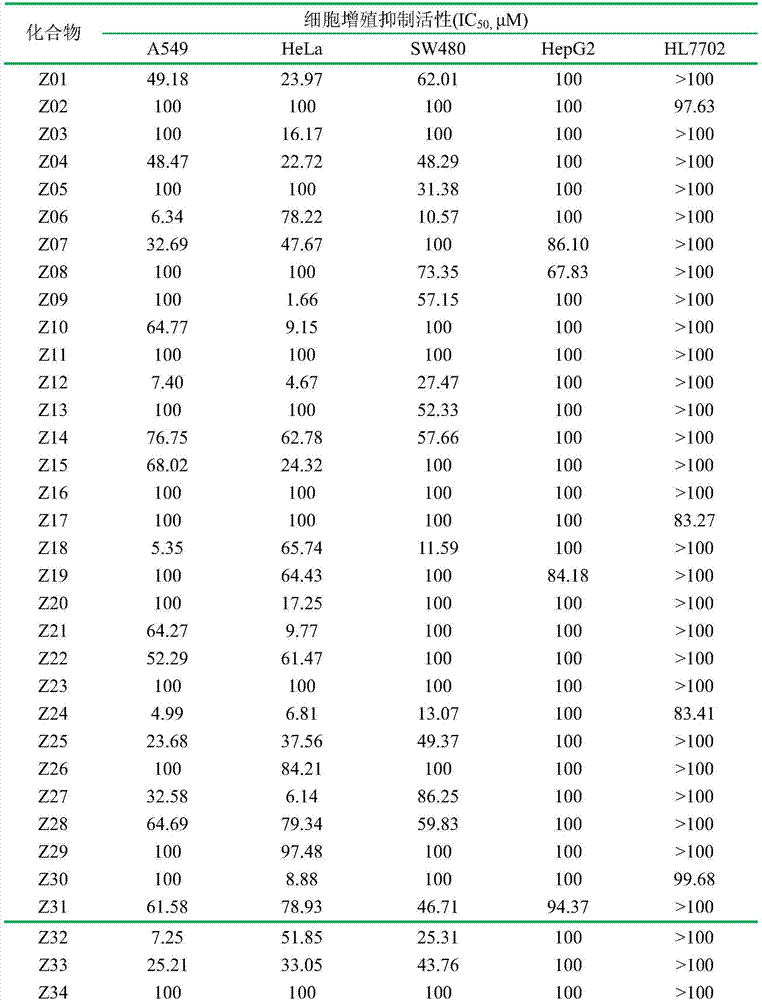
Indole carboxamide compounds and use thereof
A formamide and compound technology, applied in organic chemistry, drug combination, medical preparations containing active ingredients, etc., can solve the problems of reduced bioavailability, prolonged cardiac QT interval, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Synthesis of 3-substituted amino-2-butenoic acid ethyl ester
[0042] General synthesis method: put ethyl acetoacetate (15.01g, 0.12mol) in a 100mL eggplant-shaped bottle, slowly add 0.36mol of substituted amine aqueous solution dropwise under stirring, continue to react at room temperature for 3h after the dropwise addition, and let stand to separate layers , the organic phase was washed once with water, dried over anhydrous sodium sulfate, filtered, and used for the next step.
[0043] (1) Synthesis of 3-methylamino-2-butenoic acid ethyl ester
[0044] According to the general method of synthesis, 15.08 g of light yellow liquid was obtained, yield: 91.4%.
[0045] (2) Synthesis of 3-ethylamino-2-butenoic acid ethyl ester
[0046] According to the general method of synthesis, 16.31 g of colorless liquid was obtained, yield: 86.5%.
Embodiment 2
[0047] Example 2: Synthesis of ethyl 1-alkyl-2-methyl-5-hydroxyl-1H-indole-3-carboxylate
[0048] Synthetic general method: put p-benzoquinone (11.89g, 0.11mol) and acetone 120mL in a 250mL eggplant-shaped bottle, add dropwise under stirring to obtain the intermediate 3-substituted amino-2-butenoic acid ethyl ester 0.11 mol, after the dropwise addition was completed, the temperature was controlled at 30°C to continue the reaction for 2 h, the solvent was evaporated, and the crude product acetone was recrystallized.
[0049] (1) Synthesis of ethyl 1,2-dimethyl-5-hydroxy-1H-indole-3-carboxylate
[0050] According to the general synthesis method, 18.92g of white solid was obtained, yield: 73.7%, M.p.: 208-210°C (lit.: 208-209°C). ESI-MS, m / z: calcd.233.11 (M + ); found 234.1([M+H] + ),356.1([M+Na] + ).
[0051] (2) Synthesis of 2-methyl-1-ethyl-5-hydroxyl-1H-indole-3-carboxylic acid ethyl ester
[0052] According to the general synthesis method, 20.89g of yellow solid was ob...
Embodiment 3
[0053] Example 3: Synthesis of 1-alkyl-2-methyl-5-hydroxyl-1H-indole-3-carboxylic acid
[0054] Synthetic general method: put 0.043mol of ethyl 1-alkyl-2-methyl-5-hydroxy-1H-indole-3-carboxylate, sodium hydroxide (17.15g, 0.43mol), 100mL of ethanol and 50mL of water into In a 250mL eggplant-shaped bottle, heat to reflux for 12 hours, cool the reaction solution to room temperature, adjust the pH to 2 with 6mol / L hydrochloric acid aqueous solution, a large amount of solid precipitates, filter with suction, and recrystallize the crude product with ethanol.
[0055] (1) Synthesis of 1,2-dimethyl-5-hydroxy-1H-indole-3-carboxylic acid
[0056] According to the general method of synthesis, 6.79g of yellow solid was obtained, the yield was 76.98%, M.p.: 216-217°C; ESI-MS, m / z: calcd.205.07 (M + ); found 204.1([M-H] - ); 1 H NMR (400MHz, DMSO-d6): δ11.76(s, 1H), 8.84(s, 1H), 7.38(d, J=2.3Hz, 1H), 7.25(d, J=8.7Hz, 1H), 6.63 (dd, J = 8.7, 2.4 Hz, 1H), 3.63 (s, 3H), 2.65 (s, 3H).
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com