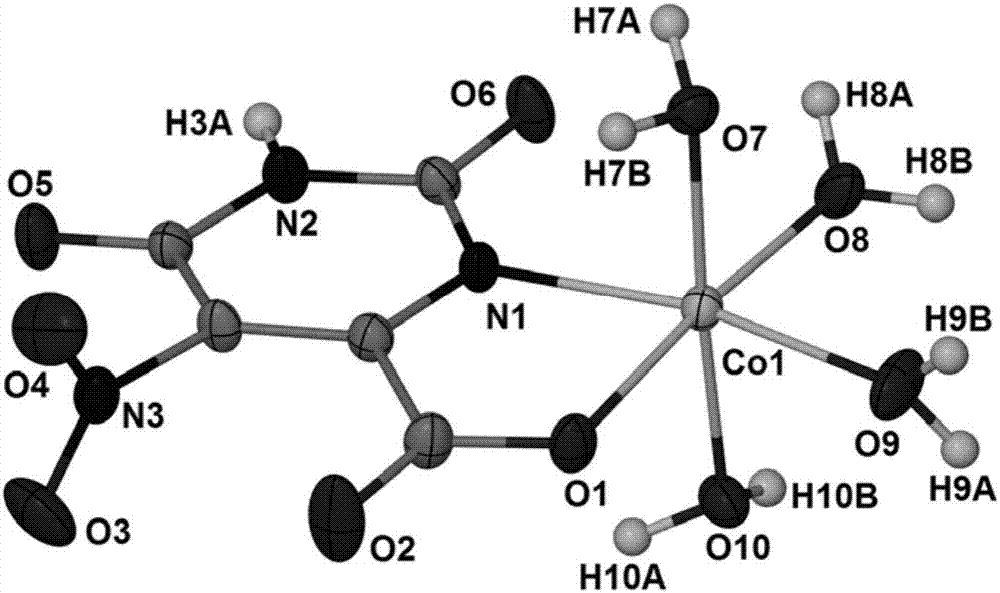
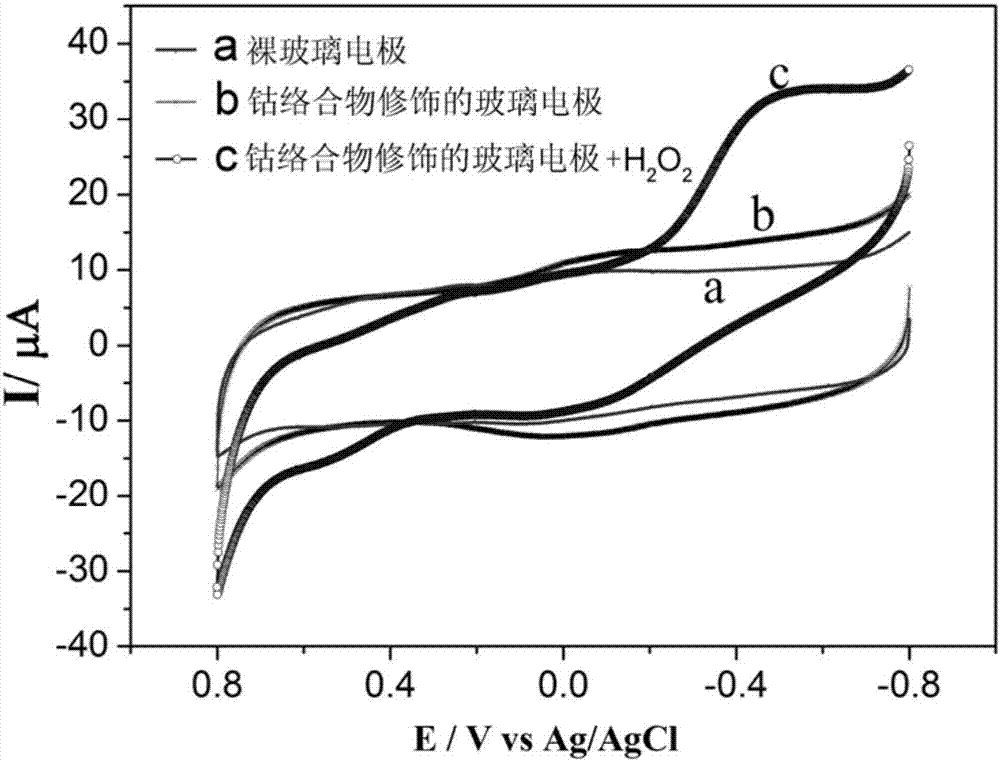
Cobalt complex electrochemically responding to hydrogen peroxide and preparation method thereof
A technology of hydrogen peroxide and complexes, applied in the field of material chemistry, can solve the problems of poor chemical stability, high price, complicated preparation procedures, etc., achieve good electrochemical response performance, and facilitate the transfer of electrons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Weigh Co(Ac) 2 ·4H 2 O (0.0747g, 0.3mmol), potassium 5-nitroorotate monohydrate (0.0772g, 0.3mmol), put in a 50mL beaker, add 15mL of distilled water, heat and stir for 20min to form a reaction mixture solution, and use hydrogen Adjust the pH value of the reaction mixture solution to 4.5 with dilute potassium oxide solution, let it stand at room temperature, slowly evaporate the solvent until crystals are precipitated, remove the residual solution by filtration, and naturally air-dry to obtain orange-yellow blocky crystals, which are cobalt complexes.
Embodiment 2
[0019] Weigh Co(Ac) 2 ·4H 2 O (0.374g, 1.5mmol), potassium 5-nitroorotate monohydrate (0.257g, 1.0mmol), put in a 50mL beaker, add 30mL of distilled water, heat and stir for 30min to form a reaction mixture solution, and use hydrogen Adjust the pH value of the reaction mixture solution to 5.5 with dilute potassium oxide solution, let it stand at room temperature, slowly evaporate the solvent until crystals are precipitated, remove the residual solution by filtration, and naturally air-dry to obtain orange-yellow blocky crystals, which are cobalt complexes.
Embodiment 3
[0021] Weigh Co(Ac) 2 ·4H 2 O (0.249g, 1.0mmol), potassium 5-nitroorotate monohydrate (0.257g, 1.0mmol), put in a 50mL beaker, add 20mL of distilled water, heat and stir for 25min to form a reaction mixture solution, and use hydrogen Potassium oxide dilute solution to adjust the pH value of the reaction mixture solution to 5.0, let it stand at room temperature, slowly evaporate the solvent to precipitate crystals, filter to remove the residual solution, and naturally air-dry to obtain orange-yellow blocky crystals, which are cobalt complexes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com